Abstract
Introduction: Red blood cell (RBC) alloimmunization occurs after exposure to non-self RBC antigens through transfusion or pregnancy. Alloimmunization rates and consequently delayed hemolytic transfusion reaction (DHTR) mortality in patients with sickle cell disease (SCD) is particularly high. Interest in a blood bank information system vendor-based alloantibody exchange in the United States (US) is growing, though cost concerns exist. To investigate the impact of an exchange that enables alloantibody data-sharing across hospitals, we conducted the first disease simulation of allo-immunized patients with SCD to project lifetime cumulative DHTR-specific mortality without an alloantibody exchange (status quo) versus with a US-wide alloantibody exchange. We then additionally performed a cost-effectiveness analysis to identify whether investing in an alloantibody exchange would be cost-effective, cost saving or neither.
Methods: We built a Markov simulation of alloimmunized patients with SCD in the US. Transition probabilities for DHTR incidence and DHTR-specific mortality were informed from the largest prospective (n=311) and retrospective (n=220) studies of DHTR incidence in people living with SCD. Age-, gender- and disease-specific background mortality probabilities were employed. Cumulative probability of DHTR was modeled from age 5, with a lifetime horizon. Costs were assessed from the health system perspective and effectiveness was calculated in quality-adjusted life-years (QALY). QALYs were informed by published lifetime simulation of patients living with SCD and matched controls. These were originally derived from a polynomial fit linking Euroqual-5 Dimensions to the visual analog scale for pain in patients with SCD in several studies. Cost-effectiveness was quantified using an incremental cost-effectiveness ratio (ICER), or net monetary benefits (NMB) if the intervention was found to be cost saving, either way incorporating a cost-effectiveness willingness-to-pay threshold of $100,000/QALY, as recommended practice. We assumed a lifetime operating cost of $10 million for an alloantibody exchange, that no patient with diagnosed DHTR has more than one DHTR recurrence, that an exchange would not be able to prevent all DHTRs (as up to 30% are not associated with a new detectable antibody in SCD), and utilized prior estimates for US allo-immunized population prevalence range of 20-44% for people living with SCD. We validated our estimate for DHTR reduction with that of the 10-year experience in the national Dutch Transfusion Register of Irregular Antibodies and Cross-match Problems (TRIX). We then conducted deterministic and probabilistic sensitivity analyses, varying all parameter estimates +/-20% and capturing uncertainty in all parameters simultaneously over 10,000 Monte Carlo simulations.
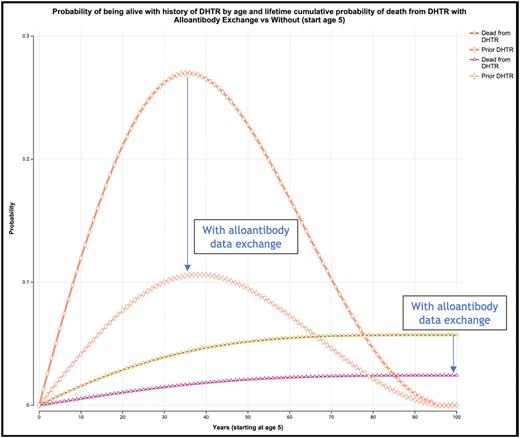
Results: Base-case lifetime simulation comparing the strategies of implementing an alloantibody exchange versus not yields a decrease in cumulative lifetime DHTR-specific mortality (2.4% vs 5.7%, respectively; Figure 1). The absolute peak probability decrease of having a history of incident DHTR and being alive occurs at age 41 (10.6% vs 27.0%; Figure 1). The strategy of alloantibody exchange implementation accrues 22.08 per-person lifetime discounted QALYs [95% credible interval 22.03-22.12] versus 21.72 without [95% CI 21.61-21.82], corresponding to a per-person QALY increase of 0.357 with exchange implementation. The lifetime population benefit for an alloimmunized population living with SCD in the United States is up to 15,710 QALYs gained. Total lifetime costs accrue $1.0 billion with exchange as compared to $2.5 billion without. This yields population-level cost savings of $1.5 billion USD ($34,091 saved per alloimmunized patient registered in the exchange), with an accrued incremental NMB of $3 billion. In probabilistic sensitivity analysis the former is favored in 100% of 10,000 Monte Carlo simulations.
Conclusion: By reducing DHTR-specific mortality, an alloantibody exchange is predicted to be a life- and cost-saving investment for alloimmunized people living with SCD. Although this analysis was limited to SCD, the impact of an alloantibody exchange would also bring benefit to patients who are alloimmunized in the context of diagnoses and states beyond SCD, such as myelodysplastic syndrome, thalassemia, autoimmune conditions, and pregnancy.
Disclosures
Hauser:Transfusion Alloantibody Exchange: Other: Founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal