Abstract
Background Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia characterized by the unique chromosome aberration t [(15;17) (q22; q21)]. APL has become highly curable with combination of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). However, ATO must be administered intravenously. Patients need long hospital stay for consolidation therapy. Realgar-indigo naturalis formula (RIF), as a traditional Chinese regimen, is an oral arsenic compound. Previous clinical trials in China proved the efficacy and safety of RIF in patients with APL. (Hong-Hu Zhu et al. Lancet Oncol 2018;871-879). The combination of RIF and ATRA created homed-based manner for APL treatment. (Hong-Hu Zhu et al. Blood 2019;597-605) However, RIF is not widely used and still only available in China. More multicenter controlled trials are needed to prove the efficacy. In addition, it is inconvenient to administer ATO/RIF 4-weeks on 4-weeks off and ATRA 2-weeks on 2-weeks off. Since the combination of ATRA and ATO induces synergistic effects, we use ATRA and arsenic both in a 2-week on 2-week off regimen to assess if it will improve curative effects.
Methods APL16 is an open-label, prospective, multicenter, noninferior, randomized clinical trial conducted in 3 hematological centers in China. We conducted this trial to evaluate whether oral realgar-indigo naturalis formula is noninferior to intravenous arsenic trioxide for non-high-risk patients with de novo APL. Eligible patients (n=81) with de novo APL have been enrolled. Patients were recruited between June 2016 and April 2022, with final follow-up in June 2022. All patients received induction therapy of ATRA 60mg/day (20-45 mg/m2/day) plus ATO (0.15 mg/kg/day). Then they were randomized to receive consolidation therapy including ATRA plus ATO or ATRA plus RIF. All patients received 6 cycles of ATRA/ATO or ATRA/RIF consolidation therapy in a 2-week on 2-week off regimen after achieving molecular complete remission. The primary end point of the study was event-free survival (EFS) at 2 years. The noninferiority margin for the difference of two arms in EFS was 5%. The secondary outcome of this trial is cumulative incidence of relapses and quality of life.
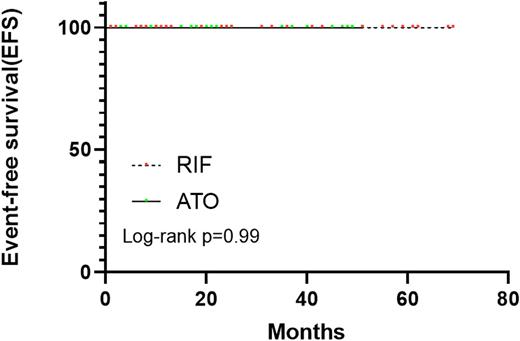
Result Enrollment of 81 patients of this study was started in September 2016 and was completed in June 2020. 6 patients were excluded including one who refused to assign the informed consent, one who had acute coronary syndrome, two who had renal insufficiency and two who died before genetic diagnosis of PML-RARA fusion gene due to intracranial hemorrhage. 75 eligible patients were randomly assigned to ATRA-ATO group or ATRA-RIF group. Two patients in ATRA-ATO group did not receive assigned treatment but received ATRA plus RIF during entire consolidation therapy. The modified intention-to-treat analysis included all 75 patients who received at least one dose of the assigned therapy after randomisation. The baseline characteristics including age, sex, white blood cell and platelet counts at diagnosis, Sanz risk and PML breakpoint were not different between the two cohorts. Until the final follow-up, 68 patients have completed their planned courses of treatment. Median follow-up for the whole cohort was 24 months. All 75 patients achieved hematologic complete remission and continued to receive consolidation therapy. Patients in both arms achieved molecular complete remission after the second cycle of consolidation therapy. No relapses and deaths have been observed. The EFS at 2 years were 100% in both groups. Differentiation syndrome occurred in nine (12%) of 75 patients during induction therapy. The incidence of decrease of fibrinogen is higher in ATRA-ATO group than ATRA-RIF group during consolidation therapy (23.5% vs 2.9%, P= .032). No difference of adverse effects except this were observed between two groups.
Conclusions: Interim results of this study proved efficacy of ATRA plus RIF was non-inferior to ATRA plus intravenous ATO for consolidation therapy in standard-risk APL. The toxicity is almost equivalent except slightly advantage of ATRA-RIF group on coagulation. No relapses may be attributed to simultaneous administer of ATRA and ATO/RIF. ATRA plus RIF offers a convenient and safe alternative for non-high-risk APL.
Trial registration: The trial was registered in the Register.ClinicalTrial.gov with NCT02899169.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal