Abstract
Introduction Replacement factor therapy for hemophilia A (HA) and B (HB), inherited disorders caused by factor VIII (FVIII) or factor IX deficiency, has significant limitations: First, factor prophylaxis is administered intravenously (IV) more than once a week. Second, patients may still experience bleeding and, third, they may develop antibodies against these therapeutic agents. A bispecific antibody, Emicizumab, a functional analog of FVIIIa, has recently been approved in patients with HA, but additional treatment options for hemophilia are still needed. We reported that genetic ablation of anticoagulant protein S (PS) improves hemostasis in HA and HB mice and constitutes a novel therapeutic target (Blood 2018, 131:1360-1371). We also showed that partial reduction of PS by small interfering RNA conjugated to an N-acetylgalactosamine cluster (GalNAc-siRNA), exclusively targeting hepatocyte-expressed PS, protects mice with HA from acute hemarthrosis. (Blood 2020, 136 (Suppl 1): 20-21).
Here, we studied hemostatic effects of partial PS reduction in human ex vivo HA models and characterized a novel GalNAc-siRNA drug candidate, SLN140, in a non-human primate (NHP) model of HA.
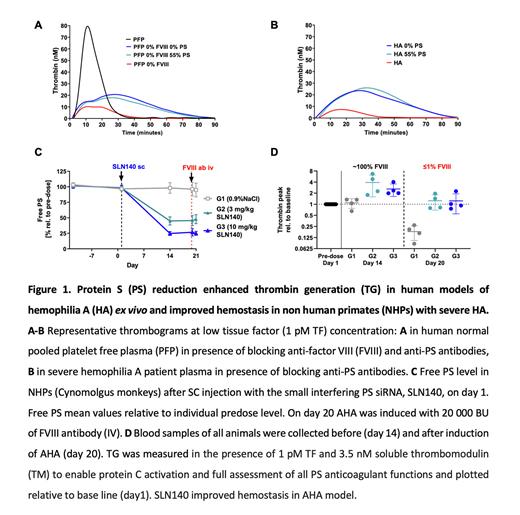
Methods & Results Hemophilia severity correlates with the amount of thrombin generation (TG) in plasma. We therefore assessed the impact of PS lowering on TG in human platelet-free plasma (PFP) with low FVIII activity. Normal and PS-depleted PFP were mixed to obtain PFP samples with free PS levels from 0 to 94%. Addition of anti-FVIII antibody resulted in PFP with FVIII between 0 and 7%. TG assays were run with low tissue factor as a trigger. Reduction of free PS to 56% doubled peak TG in PFP containing 0-7% FVIII. Further PS reduction to 37% restored peak TG in PFP containing 0-7% FVIII, indicating that partial PS depletion is sufficient to normalize TG in HA.
Since PS depletion (Stago) may also reduce tissue factor pathway inhibitor level, the next experiments were performed in normal PFP treated with anti-FVIII and anti-PS antibodies to achieve 0% residual FVIII activity and to lower free PS. Anti-FVIII antibodies lowered peak TG (10±0.1 nM vs 78±2 nM in untreated PFP). Concomitant addition of anti-PS antibodies to achieve 0% or 55% residual PS had similar impact of increasing TG peak (21±0.1 and 27±13 nM, respectively), endorsing that partial depletion of PS increases TG in PFP containing 0% FVIII (Fig 1A). These data were confirmed using severe HA patient derived-PFP. Addition of anti-PS antibodies resulted in an increase of peak TG (to 20±5 nM and to 20±8 nM at 0% and 55% free PS respectively, compared with 8±1 nM in untreated HA PFP), indicating that 45% PS reduction increased peak TG in severe HA PFP (Fig 1B).
Finally, we assessed the effect of partial PS reduction by SLN140 in a NHP model of acquired HA (AHA). TG assays were run in the presence of 3.5nM soluble thrombomodulin to enable protein C activation and full assessment of all PS anticoagulant functions. AHA was induced by slow bolus IV injection of anti-FVIII antibody (20,000 BU/kg), which resulted in <0.5% FVIII activity and a reduction in peak TG compared to controls (16±3 nM vs 43±2 nM). NHPs then received either SLN140 or 0.9% NaCl subcutaneously (SC). A single dose of 3 or 10 mg/kg SLN140 reduced free PS level from day 14 to day 21 to about 45% and 25% of baseline, respectively (Fig 1C). At day 14, peak TG relative to baseline and endogenous thrombin potential were ~3-fold higher in the SLN140-treated groups than in the 0.9% NaCl treated group. On day 20, anti-FVIII antibody was injected into all NHPs and blood was collected after 4 hours. In this AHA model, SLN140 restored TG to normal range (peak TG relative to baseline: 0.9% NaCl: 18±8%; 3 mg/kg SLN140: 124±55%; 10 mg/kg SLN140: 123±68%, Fig 1D). Treatment with SLN140 was clinically well tolerated. Basic coagulation and safety parameters were not affected by PS reduction and were comparable in the control group and the 3 and 10 mg/kg SLN140 treatment groups (mean±SD): prothrombin time 14.3±1.0, 14.4±1.0, and 14.8±1.0 seconds; fibrinogen 2.6±0.5, 2.8±0.4, and 2.4±0.6 g/L; platelet count 417±65, 436±127, and 454±112 G/L; D-Dimer 134±41, 141±59, and 188±43 ng/mL, respectively.
Conclusion PS reduction enhanced TG in human models of HA ex vivo and a single SC administration of SLN140 improved hemostasis in NHPs with severe HA. These results are encouraging for rebalancing hemostasis in hemophilia and support future clinical studies.
Disclosures
Prince:Silence Therapeutics: Research Funding. Schaeper:Silence Therapeutics PLC: Current Employment, Current holder of stock options in a privately-held company. Aretz:Silence Therapeutics GmbH: Current Employment. Eisermann:Silence Therapeutics GmbH: Current Employment. Dames:Silence Therapeutics GmbH: Current Employment. Löffner:Silence Therapeutics GmbH: Current Employment. Martinez:Silence Therapeutics PLC: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Laffan:Silence Therapeutics: Consultancy. Ahnstrom:Silence Therapeutics: Consultancy. Angelillo-Scherrer:Silence Therapeutics: Research Funding; Pfizer: Other: Speaker fees; Vaderis: Consultancy; SNSF: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal