Abstract

Background: Ascorbic acid (AA, vitamin C) deficiency is easily overlooked in the differential of patients with bruising and bleeding. While studies suggest that the incidence may be declining in the U.S in recent years, we recognized this entity clinically in the work-up of patients with unexplained bleeding or nutritional deficiencies. We analyzed AA testing and deficiency at a single institution to determine its relative incidence and associated clinical phenotype across moderate and severe deficiency.
Methods: We performed a retrospective review of MCF patients from January 1, 2018 through December 31, 2019 who underwent AA testing in a CLIA-certified standard of care laboratory following IRB approval. Deficiency was defined as an AA level ≤0.3 mg/dL. Patients were evaluated with a systematic EMR review including demographic information, presence of associated symptoms (i.e., clinical bleeding or "easy bruising or bleeding" reported on the electronic review of systems), and markers of malnutrition/malabsorption. History of hematologic disorder (e.g. sickle cell disease, thalassemia, hematologic malignancy); hematologic complications of renal disease, liver disease, solid tumor, or scleroderma; multivitamin or proton pump inhibitor use; and associated gastrointestinal (GI) disorders (i.e. inflammatory bowel disease) and gastric bypass surgery were included.
Continuous variables were summarized with the sample median and range and categorical variables with number and percentage of patients. The proportion of patients with AA deficiency was estimated along with a 95% confidence interval (CI). AA deficient patient characteristics were compared according to AA level (i.e., 0.1 mg/dL vs. 0.2 or 0.3 mg/dL) using a Kruskal-Wallis rank sum test or Fisher's exact test. P-values <0.05 were considered statistically significant. All tests were two-sided. Statistical analysis was performed using R Statistical Software.
Results: We identified 1,124 patients who underwent AA testing over 24 months, and n=245 (21.8%, 95% CI: 19.5% - 24.3%) were deficient. Of those, n=23 (9.4 %) reported symptoms of mucocutaneous bleeding (predominantly ecchymosis, easy bruising or epistaxis) and n=19 (7.8%) had GI or genitourinary (GU) bleeding. There were 42 bleeding events among 39 patients (15.9%), 3 of whom had combined mucocutaneous and GU bleeding. 100 of the deficient patients (40.8%) were taking an oral multivitamin. 171 patients (69.8%) underwent prior bariatric surgery. 79 of these (46.2%) were AA deficient before surgery.
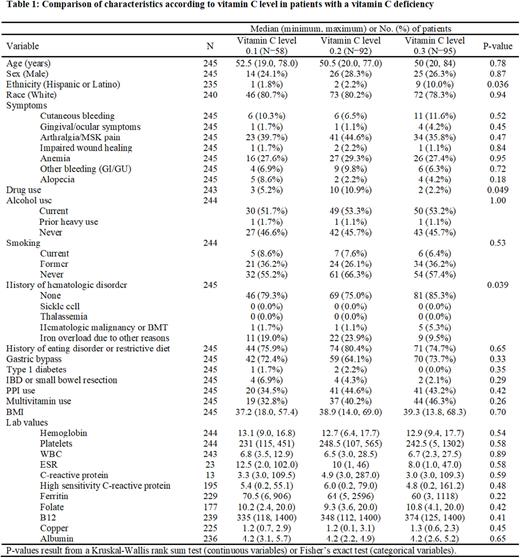
Patients were analyzed according to the degree of AA deficiency (i.e., 0.1 mg/dL vs. 0.2 mg/dL or 0.3 mg/dL, respectively) to determine the clinical characteristics associated with profound deficiency; Table 1. Significant associations were observed for ethnicity (Hispanic or Latino: 1.8% vs. 2.2% vs. 10.0%, p=0.036), reported marijuana or illicit drug use (5.2% vs. 10.9% vs. 2.2%, p=0.049), and history of underlying hematologic diagnosis, specifically predisposition to "iron overload" (19% vs. 23.9% vs. 9.5%, p=0.039). Eating disorder/restrictive diet, alcohol, gastric bypass surgery, PPI use, degree of hematologic abnormality on CBC, and other markers of nutritional deficiency were not associated with the degree of AA deficiency.
Conclusion: AA deficiency is surprisingly common but underappreciated in current practice. Nearly 22% of patients tested were deficient despite 41% of them reporting multivitamin use, and they often endorsed bleeding symptoms. We identified clinically relevant characteristics associated with the degree of AA deficiency, including drug use, hematologic disorder, and ethnicity. We found no association with risk factors like eating disorders, PPI use, and gastric bypass. While the etiology of bleeding dysfunction in this population may be multifactorial, AA deficiency likely contributes and represents a reversible cause. AA deficiency is easily confirmed and should be considered in the work-up of unexplained or easy bruising/bleeding in the absence of other clear etiologies.
Disclosures
Foran:Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities; AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal