Abstract
Monoclonal antibodies targeting CD38 (i.e., daratumumab and isatuximab) are emerging as mainstay of therapy for upfront as well as relapsed multiple myeloma (MM). These antibodies not only target CD38 on myeloma cells but also attach to CD38 antigens on non-hematopoietic tissue such as the bronchial tree causing potential Administration Adverse Reactions (AAR) during infusion/injection. This side effect is mostly explained by activation of CD38/cyclic ADP ribose (cADPR) systems contributing to airway smooth muscle tone and asthma-like symptoms (Malavasi, F, et al. Physiol Rev. 2008). After saturation of bronchial CD38 antigens, the chance of bronchospasm substantially decreases with subsequent doses. Daratumumab and hyaluronidase (DARA SC) is a subcutaneous injection approved in May 2020 for the treatment of multiple myeloma (MM). Efficacy and safety of DARA SC has been established. While clinical trials demonstrated a reduced rate of AAR with DARA SC compared to daratumumab intravenous (DARA IV), there is limited data published regarding safety of switching between the SC and IV formulation . The objective of this research was to evaluate AAR rates when switching from DARA IV to DARA SC at a tertiary medical center.
Methods: From May 1, 2020 through May 1, 2022, adult patients with MM who received DARA IV prior to DARA SC were identified through the electronic medical record a single comprehensive cancer center. The supportive medication to mitigate the AAR was the same for both preparation (acetaminophen, Diphenhydramine, corticosteroids and montelukast for patients with history of COPD or asthma). The observation for first dose of DARA SC was 6 hours as standard of care. The primary endpoint of the study was to evaluate the incidence of AAR when switching from DARA IV to DARA SC.
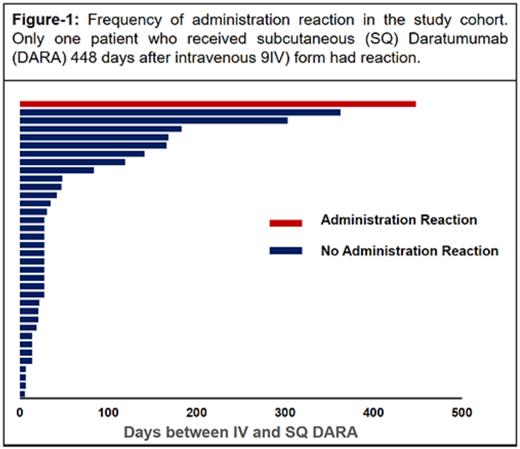
Results: 118 patients received DARA SC for treatment of MM were identified, and of those 36 patients switched from DARA IV to DARA SC. The median patient age was 70 years (range: 42-90) with majority of patients being male, N: 25 (69%). Twenty patients had Ig G MM (55%), 10 patients with Ig A (27%) and the rest, N: 6, were light chain MM (16%). The median time from the last dose of DARA IV to the first dose of DARA SC was 28 days (range:3-448). The distribution of patients based on the time interval between IV and SC preparation and AAR is shown in Figure-1. We did not detect any AAR except one patient who had grade II AAR (Erythema Face, Fever, Nausea/Vomiting, Rigors) occurred 3 hours and 36 minutes after administration of DARA SC. This patient received DARA SC 448 days after stopping DARA IV; the AARs were managed medically and patient was discharged to home and tolerated second as well as third dose without any AAR. No one else in the study cohort had any AAR to second or third dose of DARA SC.
Conclusion: A low AAR rate was observed when switching from DARA IV to DARA SC in patients being treated for MM. Our results shows no AAR by transitioning from DARA IV to SC if the time interval between the two is less than a year. Therefore, the monitoring times following first dose DARA SC after receiving DARA IV may be able to be shortened in order to increase efficiency in the infusion center and decrease chair time for patients.
Disclosures
Metheny:Gamida: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau. Malek:Sanofi: Consultancy; Amgen: Speakers Bureau; GSK: Speakers Bureau; janssen: Consultancy; BMS: Speakers Bureau; AdaptiveBio: Consultancy, Honoraria; Takeda: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal