Abstract
Introduction: Avatrombopag (AVA) is the most recent thrombopoietin-receptor agonist (TPO-RA) for treatment of adults with chronic immune thrombocytopenia (ITP; 2019) or adults with thrombocytopenia and chronic liver disease (CLD; 2018) who are scheduled to undergo a procedure. Other approved TPO-RA medications for ITP (2008) include eltrombopag (ELT), an oral medication with food restrictions, a boxed warning for hepatotoxicity, and potential need for adjustment of statin dosing; and romiplostim (ROMI), an injectable typically administered at a healthcare facility. Lusutrombopag (LUSU), an oral TPO-RA medication, was approved in 2018 for the treatment of thrombocytopenia in adults with CLD who are scheduled to undergo a procedure. AVA is an oral treatment whose bioavailability is not affected by administration with polyvalent cations (e.g., calcium, magnesium, iron) and may be administered with any food. Unlike ELT, AVA does not carry a boxed warning for hepatoxicity, nor does it require a potential dose adjustment for concomitant statins. Given its more recent approval, there is limited information in the literature on the use of AVA in real-world clinical settings. This is the first study to characterize patients initiating AVA in the US using administrative healthcare claims data and lab data.
Methods: This retrospective analysis was conducted using data from the Komodo Healthcare Map dataset. Included patients had ≥1 paid prescription for AVA between May 21, 2018 and February 28, 2022 (first such prescription was defined as the index date) and ≥6 months of closed medical and pharmacy eligibility prior to the index date (baseline period). Patients were stratified into mutually exclusive subgroups by prior evidence of approved indications (i.e., ITP, CLD, both, neither). Patient characteristics (including age, sex, and insurance type as of index date) as well as Charlson Comorbidity Index (CCI), comorbidities, and prior use of TPO-RA treatments (i.e., ELT, ROMI, and LUSU) during the baseline period were described. For patients with ≥5 years of both medical and pharmacy coverage prior to index, previous use of other TPO-RA treatments during that period was also described.
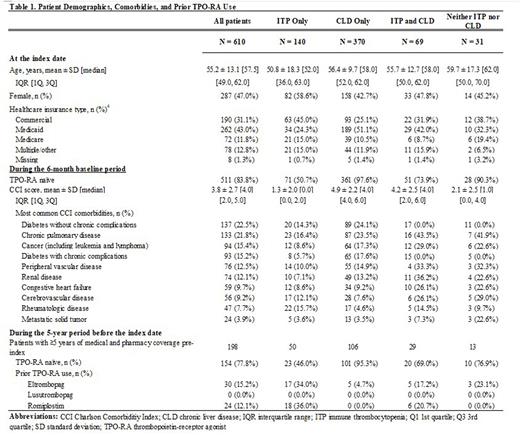
Results: A total of 610 patients initiating AVA met the above criteria. These patients included 140 (23%) with a diagnosis of ITP only, 370 (61%) with a diagnosis of CLD only, 69 (11%) with a diagnosis of both ITP and CLD, and 31 (5%) with neither a diagnosis for ITP nor CLD in the six-month baseline period (Table 1). On average, patients with a diagnosis of ITP only were 51 years of age at index date, 59% were female, and a plurality had commercial (45%) insurance coverage only. ITP patients had an average CCI of 1.3 during baseline, and the most common comorbidities were chronic pulmonary disease (16%) and rheumatologic disease (16%). 49.3% of patients with only an ITP diagnosis had been exposed to another TPO-RA within 6 months before initiating AVA treatment. Patients with CLD were, on average, 56 years of age at index date, 43% were female, and 51% had Medicaid insurance coverage only. CLD patients had an average CCI of 4.9, and common comorbidities included diabetes without chronic complications (24%) and chronic pulmonary disease (24%). Among the subgroup of ITP patients with ≥5 years of both medical and pharmacy coverage prior to index (n=50), 34.0% and 36.0% of patients previously initiated ELT and ROMI, respectively, while 46.0% were TPO-RA naïve. Among the subgroup of CLD patients with ≥5 years of both medical and pharmacy coverage prior to index (n=106), 95% had no previous use of other TPO-RA treatments; 5% previously initiated ELT, while no patients had previous exposure to LUSU or ROMI.
Conclusions: This is the first real-world characterization of patients initiating avatrombopag using data from an administrative healthcare claims database. Patients were diverse in terms of age, insurance type, and comorbidity burden. The majority of the patients administered AVA had a diagnosis for CLD; a quarter of the patients were diagnosed with ITP only. The vast majority of CLD patients were TRO-RA naïve, whereas half of the patients diagnosed with ITP had used a different TPO-RA treatment within six months before initiating AVA, indicating a recent within-TPO-RA class switch.
Disclosures
Kolodny:Sobi, Inc.: Current Employment. Oladapo:Sobi, Inc.: Current Employment. Vredenburg:Sobi, Inc.: Current Employment. Jamieson:Sobi, Inc.: Current Employment. Swallow:Analysis Group, Inc.: Current Employment. Goldschmidt:Analysis Group Inc.: Current Employment. Zichlin:Analysis Group, Inc.: Current Employment. Lopez:Analysis Group, Inc.: Current Employment. Davidson:Analysis Group, Inc.: Current Employment. Yee:Sobi, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal