Abstract
Introduction: Prolonged cytopenia is a potential side effect of CD-19 chimeric antigen receptor T-cell (CAR-T) therapy for lymphoma. ZUMA-1 demonstrated around 20% of axicabtagene ciloleucel (axi-cel) recipients had prolonged cytopenia at 3 months. Grade 3-4 cytopenia was observed in 11% of patients (Pts) at 2 years. While the mechanism behind the prolonged cytopenia is not yet well understood, one management strategy is to stimulate the bone marrow with the thrombopoietin receptor agonist, eltrombopag. This strategy is widely used in diseases like aplastic anemia and immune-mediated thrombocytopenia, but there has been limited evidence surrounding its use with cytopenia following CAR-T. Our practice is to utilize eltrombopag starting at Day 30 following CAR-T administration if either absolute neutrophil count (ANC) < 1000 cells/µL and/or if a patient is requiring growth factor support, and/or if a patient has thrombocytopenia, defined as a platelet count less than 20,000 cells/µL and/or if a patient is dependent on platelet transfusions at Day 30. We compared count recovery, progression-free survival (PFS), and overall survival (OS) of axi-cel recipients who required eltrombopag and those who did not.
Methods: A retrospective cohort of 50 adult pts treated at the University of Kansas Cancer Center who had previously received axi-cel for B-cell non-Hodgkin lymphoma between 2018-2021 were reviewed for clinical endpoints in this study. The patients were stratified into two groups based on whether they had received eltrombopag for count recovery following CAR-T administration. Pts whose disease progressed were censored at the time of progression in the analysis of outcomes. Count recovery was measured based on recovery past the lower limit of normal at our institutional lab, ANC less than 1.8 K/µL, and platelets less than 150 K/µL.
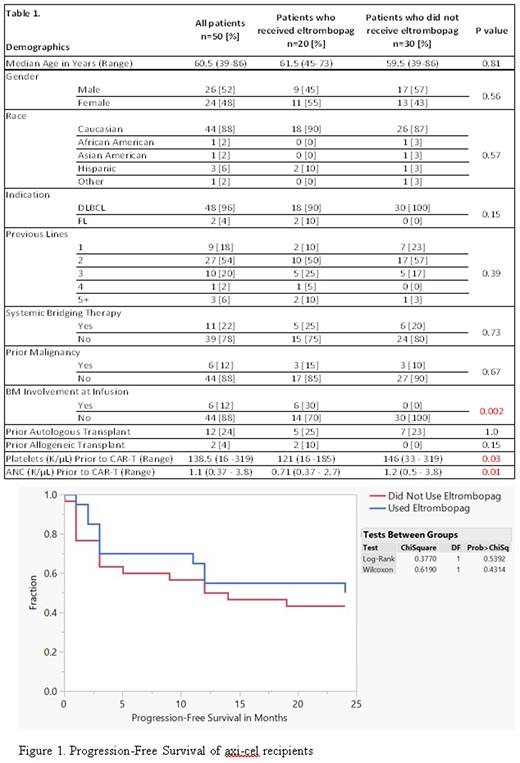
Results: Twenty (40%) pts received eltrombopag.Demographics were compared between the two groups as shown in Table 1. Patients who received eltrombopag had significantly lower platelet and ANC counts (p= 0.03 and 0.01 respectively). Those who received eltrombopag had a significantly higher incidence of bone marrow involvement at the time of axi-cel administration (p=0.002). The median duration of eltrombopag was 115 Days (9-524 days). The eltrombopag cohort continued to have significantly lower platelet (120 vs 183 K/µL, p=0.02) and ANC (1.95 vs 3.07 cells/µL, p=0.01) at 1 year. No statistically significant difference was observed in PFS or OS between patients who had received eltrombopag and those who did not, with a combined PFS of 16.5 months (p=0.54) (Figure 1) and a combined OS of 26 months (p=0.95).
Conclusion: We conclude that patients on eltrombopag had significantly lower ANC and platelet counts even at 1-year post-CAR-T. The eltrombopag group had similar OS and PFS to those who the group without cytopenia. The role of eltrombopag in the patients with prolonged cytopenia is not entirely clear and warrants further investigation, especially considering the cost of administration and risk of secondary myelodysplasia (MDS). Larger multi-center retrospective studies and prospective data of the role of eltrombopag in improving outcomes are needed.
Disclosures
Hoffmann:Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria. Abhyankar:Therakos: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau. McGuirk:Nextar: Consultancy, Honoraria; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Orca Bio: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial.
OffLabel Disclosure:
Eltrombopag is not FDA approved for post CD19 CAR-T cytopenia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal