Abstract
Background Large B-cell lymphoma has been shown to be driven by B-cell receptor signaling, which can be disrupted using Bruton tyrosine kinase (BTK) inhibition. Acalabrutinib has been studied in large B-cell lymphoma in combination with intensive therapies with encouraging results (Davies et al., Blood 2020). Preclinical studies have shown BTK inhibition could improve the effectiveness of anti-CD19 chimeric antigen receptor (CAR) T-cell therapy by improving the tumor microenvironment and delaying T-cell exhaustion (Qin et al., J Immunother 2020). BTK inhibition also has the potential to enhance the T-cell directed graft-versus-malignancy effect of allogeneic stem cell transplantation (alloHCT). This trial seeks to develop an effective therapeutic strategy to prevent relapse following cellular therapy in a specific group of large B-cell lymphoma patients that is high risk for relapse.
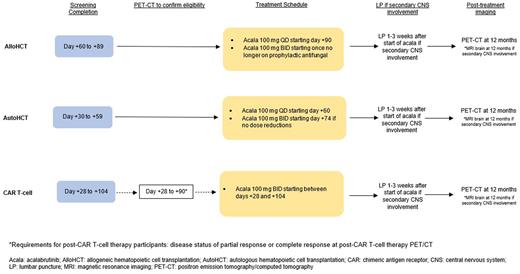
Design This is a single-arm, open-label, multi-center phase Ib/II clinical trial to evaluate the safety and effectiveness of acalabrutinib as maintenance therapy in a subpopulation of patients with large B-cell lymphoma who are at high risk for relapse after cellular therapy. Inclusion criteria includes 1) patients undergoing autologous stem cell transplant (autoHCT) or any FDA-approved CAR T-cell therapy product for high grade B-cell lymphoma, large B-cell lymphoma with a history of secondary central nervous system (CNS) involvement, histologic transformation of indolent lymphoma to large B-cell lymphoma, or large B-cell lymphoma with high-risk international prognostic index score of 4 or 5 and 2) patients undergoing alloHCT for large B-cell lymphoma. The sample size for this study is 24 patients total, including 12-15 patients treated post-CAR T-cell therapy and 12-15 patients treated post-transplant. Patients will be started on maintenance with acalabrutinib on day +90 following alloHCT, day +60 following autoHCT, or between days +28 and +104 following CAR T-cell therapy. This trial will open at University of California Los Angeles and University of California Davis (two constituent sites of the University of California Hematologic Malignancies Consortium).
Objectives
The primary objective is to determine the safety and tolerability of maintenance acalabrutinib following cellular therapy in patients with large B-cell lymphoma at high risk for relapse. Secondary objectives include to estimate the effectiveness of maintenance acalabrutinib and to estimate the rates of dose reductions, dose pauses, and permanent discontinuations of acalabrutinib that occur post-cellular therapy. Exploratory objectives include to evaluate CAR T-cell persistence with acalabrutinib maintenance, to determine if there are signs of CNS penetration of acalabrutinib, and to evaluate changes before and after initiation of acalabrutinib in intracellular cytokine and phospho-protein profiling of peripheral blood mononuclear cells.
Main Outcomes
The primary endpoint is tolerability, determined by permanent discontinuation of acalabrutinib within 12 months from cellular therapy due to intolerance, defined as grade >3 non-hematologic toxicity or grade 4 hematologic toxicity considered related to acalabrutinib that occurs for the third time after previously holding and resuming acalabrutinib. Secondary endpoints include PFS and OS following cellular therapy and the incidence of dose changes and stopping of acalabrutinib.
Conclusions This trial will use acalabrutinib as maintenance therapy for large B-cell lymphoma patients at high risk for relapse in the hopes of improving outcomes post-cellular therapy. If there is a PFS benefit in all three groups combined (CAR T-cell therapy, alloHCT, autoHCT), there will be rationale to expand each individual group into a separate larger trial. This study is registered with clinicaltrials.gov: NCT05256641.
References Davies A, Caddy J, Mercer K, et al. Acalabrutinib in Combination with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone (R-CHOP) As First Line Therapy for Patients with Diffuse Large B-Cell Lymphoma (DLBCL): The Accept Phase Ib/II Single Arm Study. Blood. 2020;136(Supplement_1):38-39.
Qin JS, Johnstone TG, Baturevych A, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother. 2020;43(4):107-120.
Disclosures
Oliai:Pfizer: Research Funding; Orca Bio: Research Funding; Jazz Pharmaceuticals: Research Funding; Arog: Research Funding; Seagen: Research Funding. Tuscano:ADC therapeutics: Research Funding; Achrotech: Research Funding; BMS: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Takeda: Research Funding; Pharmacyclics: Research Funding. Eradat:ATARA: Research Funding; Beigene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; Morphosys: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; Gilead: Research Funding; Kite: Research Funding; AstraZeneca: Research Funding; Celgene: Research Funding; BMS: Research Funding; Juno: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau. Schiller:Gamida: Research Funding; Deltafly: Research Funding; Pfizer: Research Funding; Stemline: Speakers Bureau; Janssen: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Astellas: Research Funding, Speakers Bureau; Jazz: Consultancy; FujiFilm: Research Funding; Trovagen: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Mateon: Research Funding; Geron: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Agios: Consultancy, Honoraria; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; PreCOG LLC: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Actinium: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; Ono Pharma: Honoraria; Millennium: Research Funding; Constellation: Research Funding; Glycomimetics: Research Funding; AstraZeneca: Honoraria; Kite, a Gilead Company: Research Funding, Speakers Bureau; Amgen: Current equity holder in publicly-traded company, Honoraria; Gilead: Research Funding; Medimmune: Research Funding; Arog: Research Funding; CTI: Research Funding; Cellectis: Research Funding; Regimmune: Research Funding; Cyclacel: Research Funding; Daiichi-Sankyo: Research Funding; Actuate: Research Funding; Cellerant: Research Funding; Samus: Research Funding; Stemline: Research Funding; AVM Biopharma: Research Funding; Onconova: Research Funding; Sellas: Research Funding; Deciphera: Research Funding; AltruBio: Research Funding; Genentech-Roche: Research Funding; Forma: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding.
OffLabel Disclosure:
Acalabrutinib is approved for use in mantle cell lymphoma who have received at least one prior therapy and small lymphocytic lymphoma/chronic lymphocytic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal