Abstract
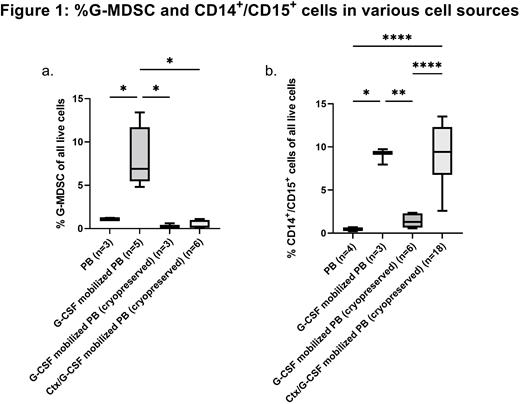
Granulocyte-colony stimulating factor (G-CSF) is used in hematopoietic stem cell transplant (HSCT) to mobilize CD34+ hematopoietic stem cells (HSC) as well as post-HSCT to speed neutrophil recovery. G-CSF mobilized grafts are enriched with two regulatory myeloid populations absent in circulation of healthy adults: granulocytic-myeloid-derived suppressor cells (G-MDSC) [CD33+CD11b+CD14-CD15+HLA-DR-LOX-1+] and cells that co-express CD14 and CD15 (CD14+/CD15+) [CD33+CD11b+CD14+CD15+]. CD14+/CD15+ cells are morphologically heterogeneous, appearing as a mix of granulocytes and monocytes. While G-MDSC and CD14+/CD15+ cells are uncommon in peripheral blood (PB), they contribute 7.2% and 9% of graft leukocytes in G-CSF mobilized PB, respectively (Figure 1). Previously cryopreserved chemotherapy (Ctx)/G-CSF mobilized grafts contain 9% CD14+/CD15+. G-MDSC are sensitive to cryopreservation (Figure 1).
G-CSF was sufficient to generate G-MDSC and CD14+/CD15+ cells via short-term culture of isolated CD34+ HSC, modelling post-HSCT immune recovery. Additional inflammatory cytokines, IL-1b and IL-6, demonstrated a synergistic effect on the production of CD14+/CD15+ cells (G-CSF: 10%, range: 4.7-20%; G-CSF + inflammation: 20.8%, range: 7.4-33.7%). There was no significant difference in percent G-MDSC produced in culture with or without the addition of inflammatory cytokines. To assess regulatory function, positively selected G-MDSC or CD14+/CD15+ cells from short term cultures were co-cultured with CD3/CD28-stimulated T cells. Both G-MDSC and CD14+/CD15+ cells suppressed T cell proliferation compared to neutrophil control (proliferative index 2.4, range: 1.4-3.4; 2.5, range: 1.5-3.8; 4.2, range: 3.1-5.8, respectively). While both cell populations produced more ROS than neutrophil controls, only G-MDSC produced arginase-I, suggesting distinct mechanisms of immune regulation.
The combination of G-CSF, a pleiotropic hematopoietic growth factor, with inflammatory cytokines induces large numbers of regulatory myeloid cells. The use of G-CSF as a mobilizing agent and post-transplant to speed count recovery results in increased numbers of regulatory myeloid cells. These cells may alter early immune recovery and graft-vs-tumor responses - and thus transplant outcomes. The clinical use of G-CSF is a potentially modifiable aspect of transplant through either use of alternative mobilizing agents (e.g., plerixafor) and avoiding empiric use of G-CSF post-transplant.
Disclosures
Wall:CRISPR/Vertex: Other: Steering committee & Institutional PI; Novartis: Other: Steering Committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal