Abstract
Purpose: The purpose of this study was to systematically identify and evaluate published literature reporting clinical and economic outcomes associated with paroxysmal nocturnal hemoglobinuria (PNH) across clinical trials and real-world settings.
Background: PNH is a rare, acquired hematopoietic stem cell disorder characterized by abnormal hemolysis of red blood cells.1,2 This complement-mediated hemolysis results in low hemoglobin [Hb] and high lactate dehydrogenase [LDH] levels, as well as symptoms including fatigue, dyspnea, pain, and hemoglobinuria.1-5 Patients frequently require costly hospitalizations and transfusions.4-10 Various measures of clinical response are used to assess treatment effectiveness and prognosis for patients with PNH, including laboratory values and transfusion dependence, but they are not consistently reported. This systematic literature review (SLR) describes clinical and economic outcomes reported in clinical trials and real-world/observational studies.
Methods: Publications were collected through systematic searches for English language studies in MEDLINE, Embase, and Cochrane libraries with no date restriction, supplemented by abstracts and associated posters from recent major conferences (2020-2021). Studies were screened for relevance by two researchers independently, with disagreements adjudicated by a third reviewer. Studies were screened at 2 levels (title/abstract and full-text) using pre-specified criteria. Clinical trials and observational studies reporting clinical response (transfusion frequency, Hb levels, LDH levels, or reticulocyte counts), healthcare resource utilization, or costs were included in this descriptive analysis. This SLR is registered with PROSPERO (CRD42022314640).
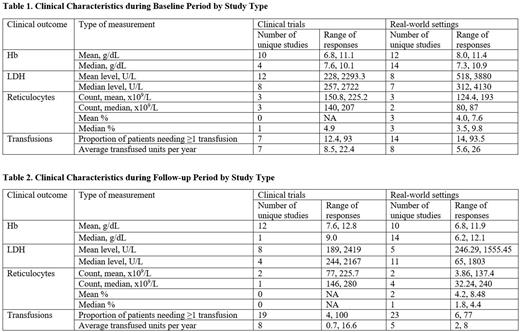
Results: Among 1607 unique publications following de-duplication, 105 studies with samples of at least 25 patients reported clinical response outcomes of interest. This included 29 clinical trials and 76 studies in real-world settings. Across these studies, methods of measuring clinical response varied: 12/29 clinical trials and 23/76 observational studies reported mean or median Hb during follow-up. These values were 11/29 and 15/76 for mean or median LDH and 4/29 and 6/76 for mean or median reticulocyte count, respectively. Mean Hb levels and mean LDH levels were the most commonly reported measures. Mean Hb levels at baseline ranged from 6.8-11.1 g/dL for patients in clinical trials and from 8.0-11.4 g/dL for patients in observational studies. Mean LDH levels ranged from 228-2293.3 U/L for patients in clinical trials and from 518-3880 U/L for patients in real-world studies. During study follow-up, mean Hb levels ranged from 7.6-12.8 g/dL and 6.8-11.9 g/dL for patients in clinical trials and real-world studies, respectively. Mean LDH levels during study follow-up periods ranged from 189-2419 U/L and 246.29-1555.45 U/L for patients in clinical trials and real-world studies, respectively. At study completion, mean and median Hb levels were <12.3 g/dL in all studies reporting values (n=35), with the exception of 1 clinical trial. Similarly, mean and median LDH levels were >225 U/L in 25/26 studies. Reticulocyte counts during study follow-up were only reported in 10 studies.
Limited data were available describing the economic burden associated with PNH. Only 3 studies reported direct costs, with total direct costs ranging from $88,251-$624,911 and PNH-related costs estimated to be $13,220. The proportion of patients who required at least 1 transfusion remained high, ranging from 4-100%.
Discussion and Conclusions: The Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation recently proposed clinical response classification based on Hb level (for complete response: ≥12 g/dL), LDH level (≤1.5 x the upper limit of normal), absolute reticulocyte count (≤150,000/µL), and transfusion requirements (none).11 This SLR has identified considerable gaps and inconsistencies in the reporting of these outcomes.
Available data reflect persistently low Hb levels and high LDH levels across both clinical trial and real-world study publications, with outcomes in real-world studies suggesting most patients have at best partial response to available treatment. These laboratory values, along with continued transfusion dependence, underscore the sustained clinical and economic burden of PNH.
Disclosures
Waheed:Novartis: Consultancy. Kaufhold:Novartis: Consultancy. Liu:Novartis: Consultancy. Lei:Novartis: Consultancy. Nellesen:Novartis: Consultancy. Geevarghese:Novartis: Current Employment. Yen:Novartis: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal