Abstract
Background Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired hematologic condition associated with significant morbidity, mortality, and impact on health-related quality of life (HRQoL). In the past, the treatment paradigm for PNH consisted of blood transfusions and treatment with complement component C5 inhibitors (C5-is), eculizumab or ravulizumab. Pegcetacoplan was approved in the US and EU in May 2021 and December 2021, respectively, and is the first approved C3 inhibitor (C3-i) for US adults with PNH and EU adults with PNH who remain anemic despite stable C5 inhibitor treatment for ≥3 months. The objective of this study was to characterize the impact of pegcetacoplan on PNH symptom incidence, frequency, and severity in comparison to C5-is in the Voice of the Patient.
Methods This mixed-methods study enrolled consenting, adult patients (>21 years old) with PNH in the US for telephone depth interviews. Eligible participants were required to be currently treated with and have at least 3 months of treatment experience with pegcetacoplan. Symptoms were described on a 7-point Likert scale (where 1 is "infrequent" or "very mild" and 7 is "always" or "very severe").
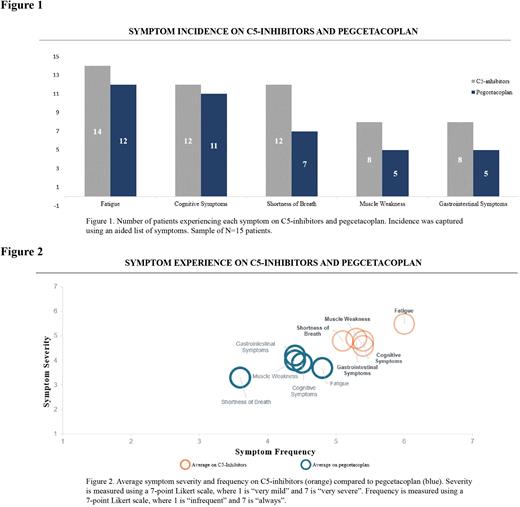
Results Results from 15 patients (8 female and 7 male) are reported here. Enrolled patients had a median age of 40 years (range 24 - 77) and all had received treatment with a C5-i prior to switching to pegcetacoplan. On average, patients had 5.6 months of pegcetacoplan experience following an average of 29.3 months of C5-i experience. The majority of patients (n=14) reported experiencing PNH symptoms while on C5-is, most notably fatigue (n=14), cognitive symptoms (n=12), shortness of breath (n=12), muscle weakness (n=8), and gastrointestinal problems (n=8). In addition to continuing to experience these PNH symptoms, patients described experiencing moderate to high symptom frequency and severity, with fatigue estimated as a 6.0 and 5.5, respectively, on a 7-point Likert scale, cognitive symptoms as a 5.4 and 4.8, shortness of breath as 5.1 and 4.8, muscle weakness as 5.4 and 4.6, and GI symptoms as a 5.3 and 4.9.
After switching from C5-is to pegcetacoplan, fewer patients reported experiencing these residual PNH symptoms, including fatigue (n=12 on pegcetacoplan vs n=14 on C5-is), cognitive symptoms (n=11 vs n=12), shortness of breath (n=7 vs n=12), muscle weakness (n=5 vs n=8), and gastrointestinal problems (n=5 vs n=8) (Figure 1). While PNH symptoms persisted on pegcetacoplan, patients reported reduction in symptom frequency and severity, including fatigue (-21% and -33% relative reduction, respectively), cognitive symptoms (-16% and -18% relative reduction), shortness of breath (-30% and -31% relative reduction), muscle weakness (-18% and -14% relative reduction), and gastrointestinal problems (-16% and -14% relative reduction) compared to their experience on C5-is. Select patients, however, reported onset of new symptoms after switching to pegcetacoplan, including shortness of breath (n=1/15) and muscle weakness (n=1/15), though these were reported as infrequent (1.0 and 2.0, respectively) and very mild (1.0 and 1.0, respectively). Patient-reported symptom experience on C5-is and pegcetacoplan is summarized in Figure 2.
Conclusions This study revealed that patients continue to experience residual PNH symptoms while on C5-is and describes improvement of patients' symptom management as it relates to incidence, frequency, and severity after switching to pegcetacoplan. Characterizing the real-world experience of PNH patients on therapy is essential to optimizing treatment options and tailoring therapies to the needs of patients.
Disclosures
Desai:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Dingli:Apellis Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Alexion Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Sanofi S.A.: Consultancy; Janssen Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal