Abstract
BACKGROUND: Febrile neutropenia (FN) and subsequent infection related mortality and morbidity remains a challenge during treatment of haematological malignancies and Hematopoietic Stem Cell Transplantation (HSCT). Opportunistic fungal infections and multi drug resistant(MDR) bacterial infections remain important causes of morbidity and mortality in neutropenic individuals. Historically from 1960 onwards granulocyte collection and transfusions for neutropenic patients have been used to treat febrile neutropenia. Previous studies to assess the role of granulocyte transfusion(GT) as bridging therapy before neutrophil engraftment have been inconclusive . Most of these studies had an absolute neutrophil coun(ANC)<0.5 X 10^9/l as inclusion criteria. Our hypothesis was that the benefit of GT may be in the subset of patients with ANC < 0.2 X 10^9/l. The objective was to analyse the outcomes of two retrospective cohorts, one with and another without GT .
METHODS: Electronic medical records of patients more than 18 years of age and undergone Induction therapy for AML and Allogenic HSCT at the institution between 2015 to 2022 were screened. Patients who developed FN with ANC < 0.2 x 10^9/l were included and those with higher ANCs at the time of GT were excluded .Patients with age less than 18 years ,those with other haemato-oncologic malignancies and patients who underwent AML induction with regimen other than 7 days of cytarabine + 3 days of daunorubicin (7+3)were excluded. Apheresed GT was available during 2020-2022 period and was used as per treating physician's discretion. Those who developed FN but having lung infection were also excluded . Patients who met the same inclusion and exclusion criteria who developed FN but did not receive GT (2015-2019 period ) were analysed as control group. Granulocytes were collected after administration of 450mcg of Granulocyte Colony Stmulating Factor (GCSF) and oral Dexamethasone 8mg 12 hours prior to collection to ABO group matched donors. Apheresis was done using Spectra Optia Apheresis System. GT was continued till infection was controlled and there were robust signs of bone marrow recovery. Forty two day survival, total duration of neutropenia and hospital stay between the two groups were compared. Data was entered in Microsoft excel version 2019 and analysed using SPSS version 21. Data were compared between two cohorts using Chi-square test and t-test for equality of means.
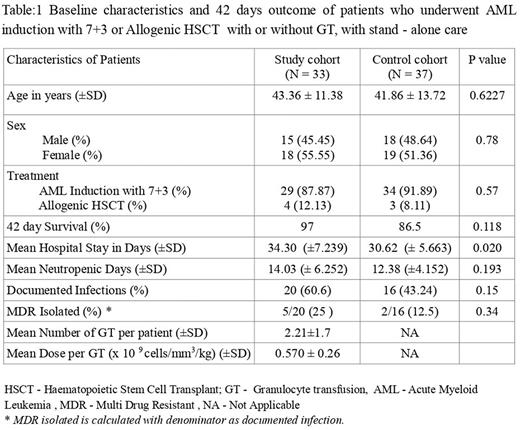
RESULTS:The demographic details of the the two cohorts is given in Table 1.Among 33 patients who received GT, 1 patient expired (3%). There were 5 patient deaths in the control cohort (n=37, 13.5%). Even though 10.5 % difference in the 42 day survival between the control and the study cohorts was noted, Chi-square analysis did not show a statistically significant difference (p =0.118). Mean duration of hospital stay for the study cohort was 34.30 days (±7.239) as compared to 30.62 days (± 5.663) for the control cohort (p = 0.020). Mean days of neutropenia for study cohort was 14.03 days (± 6.252) and 12.38 days (±4.152) for the control cohort (p=0.193).There were no grade III/IV adverse events attributable to the GT documented in the medical records of the subjects in the study cohort.
CONCLUSIONS: Although patient deaths were lower in the study cohort (with a 10% difference from the control cohort), it did not achieve statistical significance. This might have been due to the limited number of patients in both the cohorts.The study is also limited by the retrospective design.Since the study cohort and the control cohort received treatment in two different time periods, there could have been a difference in the supportive care . Inspite of all these limitations our findings may point towards the need for conducting a prospective Randomised controlled trial in patients with very severe neutropenia (ANC<0.2x 10^9/L) and other potential risk factors for MDR infections.
Disclosures
Sidharthan:Astra Zeneca: Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Emcure: Consultancy; jansen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal