Abstract
INTRODUCTION: Axicabtagene ciloleucel (axi-cel) was granted accelerated US Food and Drug Administration (FDA) approval in early 2021 for the treatment of adults with relapsed/refractory (r/r) follicular lymphoma (FL) after 2 or more lines of systemic treatment. Due to limited availability of efficacious therapies in third line and beyond, FL patients are commonly re-treated with conventional therapies. FL patients in the third line of treatment face a median progression-free survival (PFS) of 11 months, demonstrating the need for treatment options that lead to long-term remission. The objective of this study was to assess the cost-effectiveness of axi-cel under a range of model assumptions compared to standard of care (SOC) treatments in r/r FL patients who have had at least two lines of prior therapy from a US third-party payer perspective.
METHODS: A cost-effectiveness model was developed in Microsoft Excel using a partitioned-survival approach with three mutually exclusive health states: progression-free (PF) disease, progressed disease (PD), and death. Patient-level analyses of the 36-month ZUMA-5 (axi-cel) and SCHOLAR-5 (SOC) studies were used to extrapolate PFS and overall survival (OS) over a lifetime horizon. The base case analysis assumed 25% of the population alive at 5 years experienced long-term remission (i.e., functional cure); this assumption was informed by clinical opinion and was varied in scenario analyses. The remaining 75% of patients were modeled according to the best-fitting parametric survival curves identified by Akaike information criterion and the Bayesian information criterion, which were exponential models for both PFS and OS. Unit costs were obtained from published list prices and peer-reviewed literature. Healthcare resource utilization and quality of life data were obtained from published literature. Adverse event rates were obtained from clinical trial data and product prescribing information. The primary model outcome was the incremental cost-effectiveness ratio (ICER) measured as incremental cost per quality-adjusted life year (QALY) gained. In one-way sensitivity analysis (OWSA), key model parameters were varied by 20% of their base case values, or reported standard errors if available, to test their impact on the ICER. In probabilistic sensitivity analysis (PSA), all inputs for the model base case were varied by 10% or according to their reported standard errors to evaluate the impact of simultaneous uncertainty of model parameters on the ICER. Following US cost-effectiveness standard practice, costs and outcomes were discounted at 3% per year.
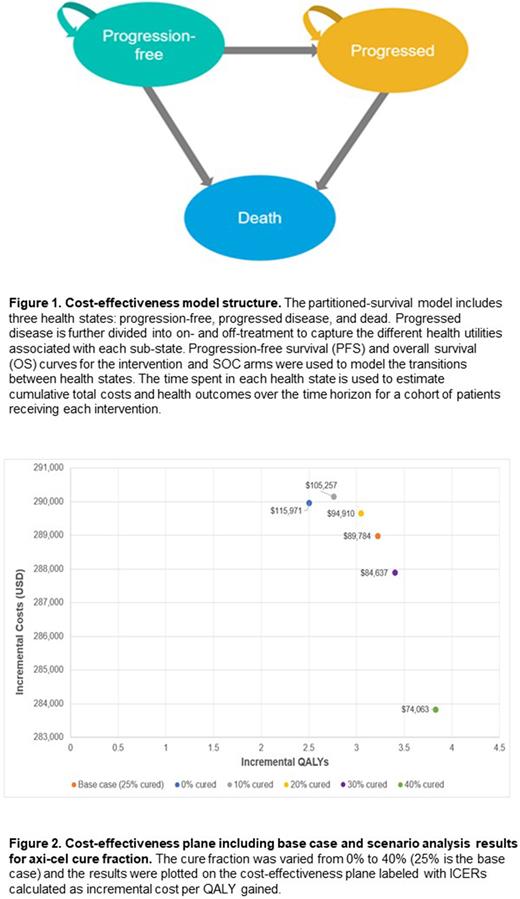
RESULTS: In the base case analysis (25% of patients alive at 5 years deemed to be functionally cured), axi-cel led to an increase of 3.92 life-years (LYs) and 3.22 QALYs relative to SOC. Axi-cel resulted in a total cost increase of $288,979 relative to SOC, driven primarily by increased PF treatment costs of $297,270. Axi-cel was also associated with reduced costs in the PD state (-$18,999) due to less time spent in PD relative to SOC. The base case ICER for axi-cel versus SOC was $89,784 per QALY gained. In scenario analyses with cure fractions ranging from 0% to 40%, the ICER varied from $115,971 to $74,063 per QALY gained. Across all parameters varied in the OWSA, the ICER varied between $122,892 and $78,628; the ICER was most sensitive to the PF health state utility, mean patient age, physician visit costs, and the length of hospitalization for axi-cel infusion. In the PSA, axi-cel had a 96% probability of being cost-effective across 1,000 iterations using a $150,000 willingness-to-pay threshold (WTP) threshold.
CONCLUSIONS: Under a range of cure assumptions, including cure fractions varying from 0% to 40%, axi-cel for treatment in r/r FL patients who have had a least two lines of prior systemic therapy would be considered cost-effective compared to SOC using the commonly cited $150,000 US WTP threshold. Results were robust across a range of sensitivity analyses accounting for parameter uncertainty. Long-term follow-up is necessary to reduce uncertainties about the proportion of patients receiving axi-cel who experience long-term remission. Additionally, given there are several new effective FL therapies in trials and pending FDA approval that were not incorporated into SCHOLAR-5, future studies should evaluate the cost-effectiveness of axi-cel against a comparator arm that includes these new treatments.
Disclosures
Oluwole:Pfizer: Consultancy; Kite, a Gilead Company: Research Funding; Novartis: Consultancy; ADC Therapeutics: Consultancy; Janssen: Consultancy; TG Therapeutics: Consultancy; Curio Science: Consultancy. Ray:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Rosettie:Kite, A Gilead Company: Consultancy. Ball:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Jacob:Kite, A Gilead Company: Consultancy. Bilir:Kite, A Gilead Company: Consultancy. Patel:Kite, A Gilead Company: Current Employment, Current holder of stock options in a privately-held company. Jacobson:Ispen: Consultancy, Honoraria; Nkarta: Consultancy, Honoraria; Clinical Care Options: Speakers Bureau; Celgene: Other: Travel Support; Humanigen: Consultancy, Honoraria, Other: Travel Support; Pfizer: Other: Travel Support, Research Funding; bluebird bio: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Daiichi Sankyo: Consultancy, Honoraria; ImmPACT Bio: Consultancy, Honoraria; Axis: Speakers Bureau; AbbVie: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Lonza: Consultancy, Honoraria, Other: Travel Support; Novartis: Consultancy, Honoraria, Other: Travel Support; BMS/Celgene: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal