Abstract
Background CD19-targeted chimeric antigen receptor-T (CART19) cell therapy has shown dramatical efficacy in B-cell lymphoma and leukemia. However, how to prolong duration of remission and mitigate the toxicities, like cytokine release syndrome, remains its obstacles. Bruton's tyrosine kinase inhibitors (BTKi) are widely used in lymphoma, and could function on T and myeloid cells. Ibrutinib, the first BTKi, has shown synergism in CART19 cell therapy. While the combined effect of higher selective BTKi, Zanubrutinib (ZB) and orelabrutinib (OB), remains unknown. Here we systematically explore the effects of three BTKi on T cells, CART19 cells and tumor microenvironment.
Method T cells stimulated with CD3/CD28 and CART19 cells were cultured in the presence of the three BTKi for 4 days and 14 days. The population doublings and cell viability were monitored, and the activation and exhaustion phenotypes (CD25, CD69, PD-1, TIM-3, CTLA-4) were analyzed by flow cytometry (FCM). CART19 cytotoxicity against NALM-6 cells were assessed for 24-hour coculture. Irradiated CD19-K562 cells were added to CART19 cell twice in 4 days with supplement of three BTKi. CART19 cells were then harvested for assessing cytotoxicity and mRNA expression. Subcutaneous tumorigenic model was established in NOD-Prkdcscid Il2rgem1/Smoc(M-NSG) mice to evaluate the synergistic effect in vivo. CART19, NALM-6 and macrophages differentiated from THP-1 upon PMA stimulation were cocultured, and IL-6 and TNF-α in the supernatants was detected by cytometry bead assays. Subcutaneous tumorigenic model was established in BALB/c mice to evaluate the effect of BTKi on tumor microenvironment. Three BTKi were delivered by oral once daily for 28 days.
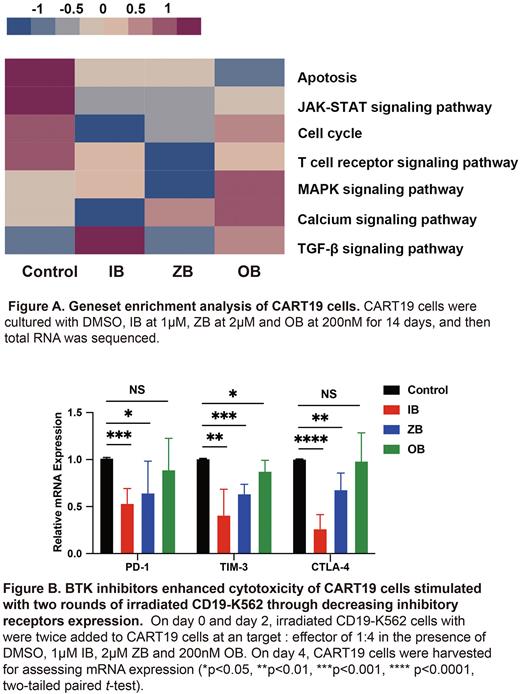
Results Three BTKi addition for 4 days and 14 days downregulated the expression of activation and inhibitory receptors on T cells to varying degrees. IB-supplement for 4 days and 14 days both decreased activation and inhibitory receptors expression on CART19 cells. The population doublings of T cells and CART19 cells were mostly increased in the addition of IB for 14 days, which could be attributed to improved cells viability (T cells: P=0.0005; CART19 cells: P=0.0059). IB combination for 14 days enhanced CART19 cells cytotoxicity. RNA sequence highlighted BTKi downregulated T cell activation associated signaling pathways, like JAK-STAT (P=0.0299) (Fig. A). All BTKi improved cytotoxicity of CART19 cells exposed to serial irradiated CD19-K562 cells (IB: P<0.0001; ZB: P<0.0001; OB: P<0.0001). qRT-PCR analysis showed BTKi decreased mRNA expression of PD-1, TIM-3 and CTLA-4 (Fig. B). In subcutaneous tumorigenic model, bioluminescence imaging monitoring showed tumor burden was alleviated in IB group (P=0.0274). IB and OB increased CART19 cells proportion in tumor tissue and bone marrow on day 28. In tumor microenvironment model ex vivo, BTKi downregulated the levels of IL-6 (IB: P=0.0270; ZB: P=0.0629; OB: P=0.0028) and TNF-α (IB: P=0.0250; ZB: P=0.0578; OB: P=0.0981). In the subcutaneous lymphoma model, the three BTKi reduced tumor-infiltrating macrophages (IB: P=0.0158; ZB: P=0.0186; OB: P=0.0554) and impeded type 2 macrophages (IB: P=0.0061; ZB: P=0.0079; OB: P=0.0262).
Conclusion In summary, our data reveal that BTKi prevent strong activation, sustained tonic signaling and serial antigen-specific stimulation. BTKi administration impairs macrophages secreting IL-6 and TNF-α ex vivo, and reduces tumor associated macrophages in vivo. We provide evidence that BTKi combination is a potential strategy to improve CART19 cell therapy by modulating immune system.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal