Abstract
Chimeric Antigen Receptor (CAR) T cell therapy is arguably one of the most significant breakthroughs in cancer treatment. There are currently six FDA-approved products that are commercially available, and they have shown remarkable clinical results. However, one of the main challenges is the persistence of the transduced effector cells so they can exert a long-term durable response. Therefore, strategies that increase the persistence of CAR-T cells constitute an unmet need in cellular therapy development.
We sought to address this important problem by optimizing anti-ROR1 CAR constructs. ROR1 is a relevant tumor-associated antigen expressed in multiple hematological malignancies and solid tumors. Initially, we optimized the scFvs "front-end” binding domain using computational tools for codon optimization, positional arrangement of heavy-light chains, and linker length evaluation. The second optimization step included the comparative assessment of the "back-end” signaling domains of the second (2G) and third-generation (3G) CAR construct. The selection of the co-stimulatory combination domain was based on this: The ICOS co-stimulatory domain has shown an augmented effector function and in vivo persistence of Th17 polarized cells compared with CARs with CD28 or 4-1BB intracellular domains. On the other hand, OX40 signaling enhanced CAR-T cells’ cytotoxicity and reduced exhaustion markers, thereby maintaining their function in immunosuppressive tumor microenvironments. Therefore, in this study, we evaluated the combination ICOS-OX40 as a novel 3G approach compared to the previously published combination CD28-BBζ, ICOS-BBζ, and a second generation using BBζ as co-stimulatory domains.
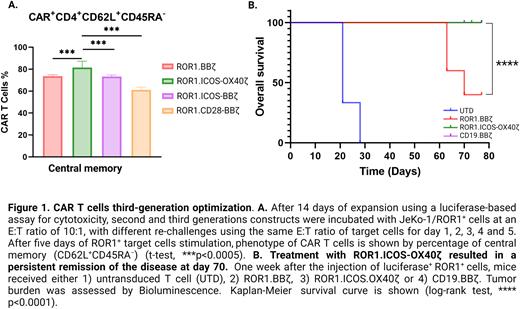
Using luciferase-based cytotoxicity assays, we observed that 3G constructs induced higher levels of apoptosis in ROR1+ cell lines for lymphoma (Mantle Cell Lymphoma cell line JeKo-1). Furthermore, repetitive antigen challenge in vitro also demonstrated better fitness, suggesting improved persistence and less exhaustion of 3G constructs than the 2G ones. Moreover, the 3G-ICOS-OX40ζ construct induced T cells with a higher central memory phenotype (Figure 1. A) with similar exhaustion markers compared to other 3G constructs.
To verify the significance of our in vitro findings, we investigated the impact on the persistence and exhaustion of ROR1 2G vs. 3G CAR constructs in vivo using a NOD-SCID-γ-/- (NSG) mice model engrafted with 0.5x106 luciferase+ ROR1+ Jeko-1 cells. Tumor-bearing mice were treated with a single intravenous (IV) injection of 3x106 CAR-T cells, including 2G, 3G constructs, untransduced T cells, PBS vehicle, and CAR-CD19 as controls. Tumor burden changes were monitored regularly by bioluminescent imaging. Treatment with ROR1.CAR-T cells led to a durable remission and long-term survival (Figure 1. B). Furthermore, on day 70 post-infusion, the 3G-ICOS-OX40ζ induced better remission of the disease compared to the 2G-construct and showed increased levels of CAR-T cells, suggesting improved persistence, a relatively low expression of exhaustion markers, and an increased number of CAR-T cells with a central memory profile.
Our results demonstrate that systematically optimizing the "back-end” signaling CAR construct 3G domains enhances T cell effector function and cytotoxicity. Furthermore, our preclinical data demonstrate for the first time the combination of 3G-ICOS-OX40ζ in CAR-T cells as a novel strategy to overcome exhaustion and improve the persistence of CAR-T cell therapy in hematological malignances. 3G-ICOS-OX40ζ showed improved in vitro and in vivo cytotoxicity and persistence with less exhaustion and an increased number of central memory phenotypes. These findings are very encouraging and constitute the rationale for advancing the evaluation of this 3G construct in preclinical and clinical studies.
Disclosures
Castro:Kite Pharma: Research Funding; Fate Therapeutics: Research Funding; Tmunity Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal