In this issue of Blood, Syrykh et al report the experience of the French Lymphopath network in the use of core needle biopsies (CNB), as compared with excisional lymph node (SE) biopsies, as a tool for the primary diagnosis of lymphoma.1
The authors conclude that this methodology is reliable, overall, for most categories of lymphoma (92.3%), although lymph node excision led to a higher rate of conclusive diagnoses (98.1%). Categories that proved most problematic for accurate diagnosis and subclassification on CNB were peripheral T-cell lymphomas and Hodgkin’s lymphoma subtypes. The French Lymphopath network is a remarkable model system for providing expert review and diagnosis, and importantly facilitates accurate diagnostic information and access to pathological specimens for clinical trials and translational research. Since 2010 all French pathologists have been encouraged to submit biopsies for expert review by a panel of 64 hematopathologists. The 30 participating reference centers provide full access to ancillary immunohistochemical and molecular tests for diagnosis. This study is based on 32 285 lymph node samples, of which 10 285 were CNB and 22 000 were SE. Of note, during the study period CNB increased from 25% of specimens in 2010 to 40% in 2018. In our practice settings, trends during the recent pandemic have shown an even greater reliance on core biopsies for primary diagnosis, with reduction in in-patient admissions needed to accomplish surgical excision.
Centralized pathology review of lymphoma diagnoses for clinical trials in the United States began in 1967 with the establishment of the Pathology Panel and Repository Center for Lymphoma Clinical Studies.2 It was organized under the auspices of the Lymphoma Task Force established by the National Cancer Institute in 1964. However, this system provided only retrospective review for analysis of clinical trials, and was not a tool to facilitate accurate diagnosis for patient care. More recently, many centers have recognized the importance of timely pathology review for clinical management.3-5 The All Wales Lymphoma Panel established in 1998 indicated that 46% of cases had a change in clinical management, which was substantial in 8% of cases, as a result of central pathology review.3
Syrykh et al report a concordance rate between the referral and expert panel diagnoses of 78.2%, which was not substantially different between CNB and SE. Diagnostic categories that were challenging in both types of specimens were grade 3B follicular lymphoma, nodal marginal zone lymphomas, and lymphoplasmacytic lymphoma. Because of the need to evaluate nodal architectural features, accurate diagnosis of peripheral T-cell lymphoma and Hodgkin lymphoma often required excisional biopsy. Only 125 patients had both CNB and SE. Factors that led to SE following CNB included 1) unclassifiable lymphoma, 2) uncertainty between benign and malignant, and 3) inadequate tissue for diagnosis. However, no data are presented on cases in which a diagnosis based on CNB was ultimately revised.
Syrykh et al suggest that CNB may be suitable for diagnosis and initial treatment for the patient with newly diagnosed lymphoma. However, residual archived material from an initial diagnostic specimen may also be important in later management of the patient. For example, testing for specific mutations, such as EZH2, may be performed long after the initial diagnosis to allow for personalized therapeutic approaches. Clonotype-specific minimal residual disease testing also may require access to the primary tumor. Finally, further studies of the initial biopsy specimen for surface antigens, such as CD30 or PD-L1, may be necessary for clinical trial enrollment.
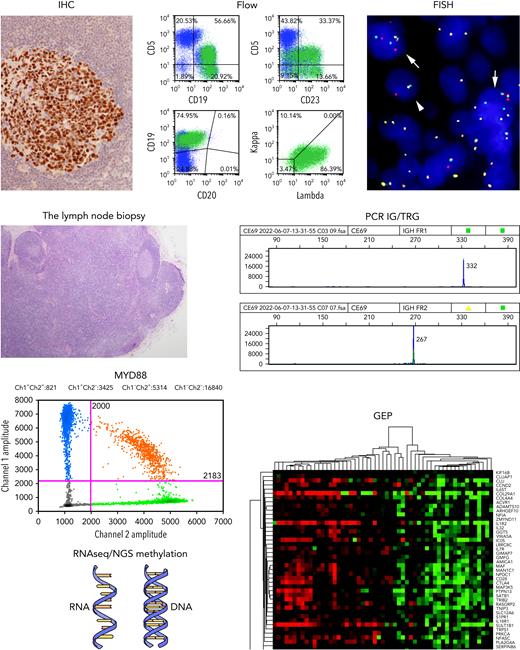
Looking beyond the management needs of the individual patient, the trend toward increased reliance on CNB raises concern about its impact on clinical research and progress in our understanding of malignant lymphomas. An extensive diagnostic armamentarium (see figure) is often implemented for an accurate diagnosis of lymphoma. CNB may yield suitable material for immediate clinical management, but is unlikely to produce adequate material for an ever-widening battery of genomic studies being used today. Additionally, future advances are dependent upon access to tumor tissue to permit the high-throughput sequencing studies that inform us about underlying biology, response to therapy, and the eventual development of targeted therapies that will lead to improvement in patient outcomes.6 For example, we know today that diffuse large B-cell lymphoma is not a single disease, and but represents a family of aggressive B-cell lymphomas with transformation via diverse genomic pathways.7,8 We are just beginning to understand the pathogenesis of many subtypes of peripheral T-cell lymphoma (PTCL). Notably, PTCL, not otherwise specified, is a heterogenous family of neoplasms at the genomic level.9
The lymph node biopsy. A variety of methodologies play a role in the contemporary diagnosis of lymphoma. These include immunohistochemistry (IHC); flow cytometry (Flow); fluorescence in situ hybridization for genomic rearrangements (FISH); PCR for immunoglobulin and T-cell receptor gene rearrangements (PCR IG/TRG); PCR for targeted single cell mutations (eg, MYD88); high-throughput sequencing methods for analysis of RNA and DNA (RNAseq/NGS Methylation); and gene expression profiling (GEP). Karyotyping performed on metaphase spreads is still valuable in some settings.
The lymph node biopsy. A variety of methodologies play a role in the contemporary diagnosis of lymphoma. These include immunohistochemistry (IHC); flow cytometry (Flow); fluorescence in situ hybridization for genomic rearrangements (FISH); PCR for immunoglobulin and T-cell receptor gene rearrangements (PCR IG/TRG); PCR for targeted single cell mutations (eg, MYD88); high-throughput sequencing methods for analysis of RNA and DNA (RNAseq/NGS Methylation); and gene expression profiling (GEP). Karyotyping performed on metaphase spreads is still valuable in some settings.
Groups such as the Lymphoma Leukemia Molecular Profiling Project are dependent upon a carefully annotated tumor bank integrated with clinical information and outcome data.10 CNB may yield sufficient material for initiation of therapy, but is unlikely to allow banking of tumors for future studies. Thus, we would urge the continued use of surgical excision for primary diagnosis, especially for patients being enrolled in clinical trials. Continued access to biopsy material is necessary to sustain progress in our field.
Conflict-of-interest disclosure: None of the authors has a relevant conflict of interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal