In this issue of Blood, Feurstein et al1 elegantly demonstrate, for the first time, that nearly 7% of myelodysplastic syndrome, also termed myelodysplastic neoplasm (MDS), and chronic myelomonocytic leukemia (CMML) patients carry a pathogenic or likely pathogenic (P/LP) germ line variant associated with germ line predisposition disorders (GPD). Widespread adoption of next-generation sequencing (NGS) in myeloid malignancies has unraveled a gamut of genes implicated in GPD, recognized by both the World Health Organization and International Consensus Classifications.2,3 GPD is enriched in younger patients with MDS, but it is also detected in older patients without a family or personal history of cancer. Despite the important management implications, the frequency of underlying GPD in patients with MDS is unknown.
The authors conducted this study on a large MDS cohort (n = 404) spanning all age groups (range, 1-75 years) in the CIBMTR database. The cases were selected based on (1) diagnosis of MDS/CMML, (2) underwent first allogeneic hematopoietic stem cell transplantation (HSCT) from a related donor, and (3) availability of peripheral blood (PB) samples from both the patient (recipient) and related donor. Whole-exome sequencing (with additional spike-in probes for noncoding regions of ANKRD26, DKC1, FANCI, GATA2, TERC) was performed on recipient and donor (808 samples) to identify P/LP variants in genes associated with GPD. Because the donor, in this case, was an unaffected carrier from the family, the authors used the presence of identical P/LP variants in patient and donor, or P/LP variants in the patient, reported exclusively as germ line in the literature with 40% to 60% allele frequencies (VAF) as evidence of germ line origin.
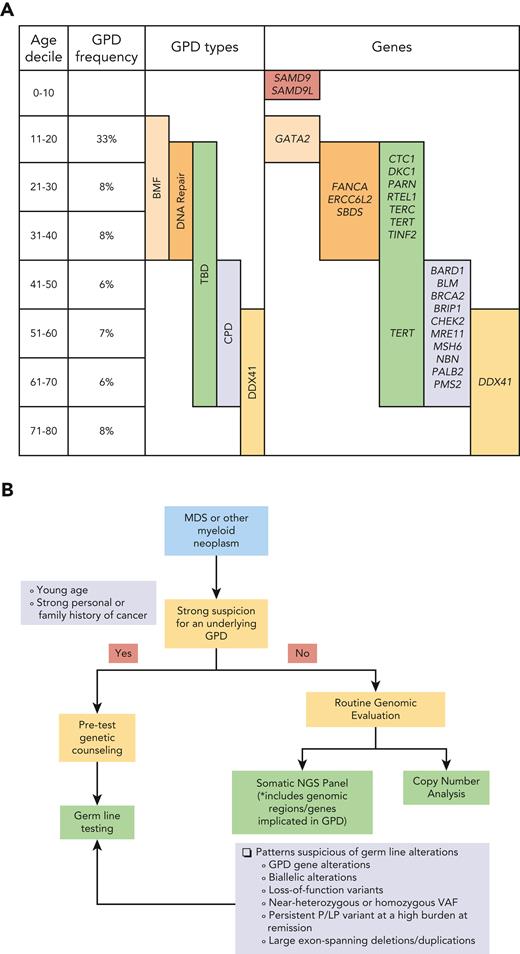
Using these stringent criteria, a total of 28 patients with MDS (∼7%) had a P/LP germ line variant. These were distributed across all age deciles, with frequencies ranging from 6% (1 in 17 patients) to 33% (1 in 3 patients). When the types of GPDs and implicated genes were evaluated, there were clear demarcations in their distribution across the age spectrum. In adult MDS patients aged <40 years, germ line P/LP variants involved genes related to DNA repair and bone marrow failure syndromes (CTC, DKC1, ERCC6L2, SBDS). In MDS patients aged >40 years, P/LP variants involved genes associated with general cancer predisposition syndromes (BRCA2, CHEK2, MSH6, PMS2). Telomere biology disorders spanned the age spectrum, while DDX41 variants were seen in older MDS patients (see figure panel A).4-6 A significantly higher proportion of MDS patients with germ line P/LP variants had bone marrow blasts ≥5%; however, there were no differences in other clinicopathologic features, IPSS-R, and outcomes, although much data was unavailable, so prospective exploration is needed. There were no apparent differences in somatic alterations between the 2 groups, but the study was done on PB (not bone marrow) samples and was limited in numbers and depth of coverage (40×).
A significant proportion (overall ∼7%) of patients with myelodysplastic syndrome or myelodysplastic neoplasm (MDS) have a deleterious gene variant implicated in germ line predisposition disorder (GPD). The frequencies, types of GPDs, and the genetic alterations vary across different age ranges. Accurate identification of patients with GPD is of utmost importance as it necessitates modification to the management approaches. In addition to unusual clinical presentations such as younger age of onset and strong personal or family history of cancer, there are specific patterns of gene alterations that can be noted on routine somatic mutation panel and copy number testing that are highly suspicious for underlying GPDs. The proposed comprehensive diagnostic work-up can provide important clues to identify these patients.
A significant proportion (overall ∼7%) of patients with myelodysplastic syndrome or myelodysplastic neoplasm (MDS) have a deleterious gene variant implicated in germ line predisposition disorder (GPD). The frequencies, types of GPDs, and the genetic alterations vary across different age ranges. Accurate identification of patients with GPD is of utmost importance as it necessitates modification to the management approaches. In addition to unusual clinical presentations such as younger age of onset and strong personal or family history of cancer, there are specific patterns of gene alterations that can be noted on routine somatic mutation panel and copy number testing that are highly suspicious for underlying GPDs. The proposed comprehensive diagnostic work-up can provide important clues to identify these patients.
Although numbers may seem low, these findings are extremely significant as detection of GPD heavily influences the management of patients. This includes a strong consideration of HSCT (if feasible) from unrelated donors or related donors prescreened for mutation as an additional step, initiation of genetic counseling, and long-term surveillance for cancer prevention and early detection for patients and unaffected carriers. Close monitoring of carriers for MDS development using multimodal approaches is needed, because it is unclear whether standard morphologic diagnostic criteria apply or if a higher threshold is required in this setting.7
From the clinical diagnostic laboratory perspective, the finding that underlying GPD is not infrequent in MDS has major implications for design and interpretation of somatic NGS panels. Germ line testing in patients with a myeloid neoplasm requires pre-test genetic counseling followed by skin fibroblast culture (which takes up to ∼6 weeks). Although routine germ line testing in every MDS patient is ideal, the process is quite onerous and may not be feasible at all centers. On the other hand, inclusion of known genomic (coding and noncoding) gene regions associated with GPDs (both hematologic and other cancers such as BRCA2, CHEK2, MSH6 as noted in this study) into routine somatic NGS panels can facilitate incidental detection of P/LP variants even in the absence of family/personal history and usual age of presentation. Suspected variants can be further investigated by germ line testing following discussions with a genetic counselor.5 The panels require frequent updating to encompass the ever-expanding list of genes, most of which have been recent discoveries even within pediatric population.8 Specific patterns of gene aberrations detected by these panels are highly predictive of GPD. These include biallelic gene alterations (multiple P/LP variants, or P/LP variant with either a copy number change or copy-neutral loss-of-heterozygosity involving the other allele), near-heterozygous or homozygous VAF, and persistence of the P/LP variant at a high burden at follow-up despite achieving remission. Loss-of-function variants and large exon-spanning deletions/duplications suggest a germ line origin. The latter can be missed by short-read NGS. It is important to be cognizant of these limitations of NGS and use other techniques (eg, chromosomal microarrays, long-read sequencing, multiplex ligation-dependent probe amplification) for specific GPDs suspected in that age range (see figure panel B).
The reported frequencies are still an underestimate. A subset of related individuals may not carry the germ line variant, and the HSCT donor pool would be enriched for those lacking the P/LP variants. Germ line testing could upgrade some “presumed germ line” or variant of unknown significance, but could invalidate a small proportion of P/LP variants. MDS patients with family histories of malignancy may be underrepresented owing to a preference for unrelated HSCT. Another study on acute myeloid leukemia showed that ∼30% of RUNX1 mutations were germ line.9
In summary, the study by Feurstein et al provides an insight into the frequency and spectrum of GPDs in MDS patients. The National Comprehensive Cancer Network recommends germ line testing for MDS patients <50 years old, with hypocellular marrow or extra-hematological stigmata of GPD.10 Based on a relatively high frequency of P/LP germ line variants noted in this study across all age groups, a low threshold for germ line testing in all MDS patients is warranted regardless of the above characteristics. Alternatively, inclusion of GPD genes in routine somatic NGS panels would enable identification of patients with a high pre-test probability for germ line work-up. After all, recognition of underlying GPD in MDS is an essential step for further studies to validate the diagnostic criteria, prognostic models, and therapies specific for these entities.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal