In this issue of Blood, Cichocki et al demonstrate the functionality of multiply engineered induced pluripotent stem cell (iPSC)-derived NK cells designed as immunotherapy for B-cell malignancies.1
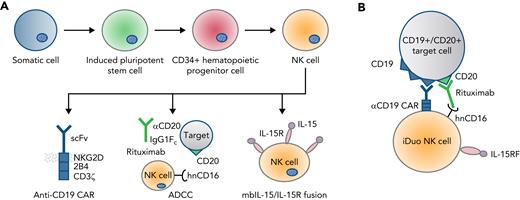
While chimeric antigen receptor (CAR)-T cells as therapy for CD19+ cancers have been markedly successful at eradicating disease, remissions are transient in a substantial number of cases and disease relapse due to antigen loss is common. To circumvent this issue, the authors triple-modified iPSC-derived NK cells to express a CD19 targeting CAR for antigen specificity, a high affinity, non-cleavable CD16 (hnCD16) to augment innate antibody-dependent cellular cytotoxicity (ADCC), and a membrane bound IL-15/IL-15R fusion protein (IL-15RF) for enhanced persistence (see figure panel A). These triple-modified cells (“iDuo” NK cells) have robust CAR-mediated antigen-specific cytotoxicity that is boosted when combined with the CD20-binding antibody, rituximab. iDuo NK cells have clear potential as an innovative cellular therapy that can be combined with therapeutic antibodies to mitigate relapse risk secondary to disease heterogeneity. Consequentially, the product has rapidly progressed to clinical testing with a phase-1 multicenter trial now actively recruiting patients (NCT04245722).
Derivation, specificity, and functionality of iDuo NK cells. (A) iDuo NK cells are genetically engineered from iPSC-derived NK cells incorporating expression of a CD19-targeting CAR with NKG2D transmembrane and 2B4/CD3ζ signaling domains, a high-affinity, non-cleavable CD16, and a membrane-bound IL-15/IL-15R fusion molecule. (B) Triple modification allows for dual target-mediated killing through the CAR with enhanced ADCC when used in combination with monoclonal targeted antibody.
Derivation, specificity, and functionality of iDuo NK cells. (A) iDuo NK cells are genetically engineered from iPSC-derived NK cells incorporating expression of a CD19-targeting CAR with NKG2D transmembrane and 2B4/CD3ζ signaling domains, a high-affinity, non-cleavable CD16, and a membrane-bound IL-15/IL-15R fusion molecule. (B) Triple modification allows for dual target-mediated killing through the CAR with enhanced ADCC when used in combination with monoclonal targeted antibody.
NK cells are lymphocytes with innate effector function that defend against infection and malignancy. Their powerful intrinsic cytotoxicity can be complemented by genetic engineering. NK cells perform their immune function through three major pathways: 1) direct cytotoxicity, 2) ADCC, and 3) secretion of inflammatory mediators. Importantly, NK cells are functionally activated through a balance of stimulatory and inhibitory signals and are not restricted by antigen presentation or HLA restriction.2 NK cells thus contribute to the inflammatory milieu but do not directly cause graft-versus-host disease3 and are less toxic than allogeneic T-cell therapies.4 While these promising attributes have fueled excitement and prompted studies of NK cell adoptive transfer, practically there remain a myriad of challenges to clinical translation. Among these are a need for standardization of clinical-grade ex vivo activation and expansion, tumor-mediated immune suppression, and limited in vivo persistence and infiltration into the tumor microenvironment.5-7 iDuo NK cells were thoughtfully designed to address these issues. An outstanding question is whether they, or future generations of engineered NK cell products, can truly overcome the limited NK cell therapeutic efficacy seen to date.
First, the authors address whether the expressed CAR and hnCD16 have additive cytotoxic functionality (see figure panel B). The dual effect is shown in cell culture and in vivo studies. As expected, iDuo NK cells mediate robust cytotoxicity against a range of CD19-expressing targets. Adding rituximab tests hnCD16-enhanced ADCC with the potent cytotoxicity observed of iDuo NK cells against even CD19− targets. Combinatorial CAR- and ADCC-mediated NK effector function was further supported using an in vitro model of tumor heterogeneity, including a mix of CD19+ and CD19− AHR-77 cells. In this model, the combination of iDuo NK cells and rituximab was highly cytotoxic, and mediated effective target elimination regardless of CD19 expression. The efficacy of the iDuo NK cell/antibody pairing was further evident in killing assays of primary CLL cells obtained from five different patients, and in an in vivo model of aggressive lymphoma. Durable remissions in treated mice were achieved with iDuo NK cells and rituximab when the cells were multiply dosed, and their persistence supported with IL-2 cytokine injections.
Although addressing tumor heterogeneity and antigen escape is important in relapse prevention, perhaps the most limiting characteristic of NK cell adoptive therapy is the relatively short circulation time of the infused cells.5,8 iDuo NK cells express IL-15RF as a strategy to support NK cell activation and persistence. However, because the included in vivo studies were completed within a relatively short timeline and/or included multi-dose administration together with serial IL-2 injections, to infer iDuo NK durability is not possible. Clinical use of systemic IL-2 at doses likely to be necessary for effective NK cell stimulation is associated with an unfavorable side effect profile with unspecific inflammation as a predominant feature. Future experimentation should test iDuo NK cell persistence in the absence of IL-2, in order to limit potential toxicity.
While the anti-tumor functionality of iDuo NK cells is impressive, clinical translation of cellular therapy products is complex. To this end, iDuo NK cells are clonally derived from a master human iPSC line. This allows product scalability and the possibility of homogenous cell-bank renewable manufacture for on-demand access without need for further engineering or enrichment. While the consistency of iPSC-NK cell manufacture is noteworthy, these cells have prior been shown to be relatively undifferentiated with high inhibitory receptor NKG2A expression and an immature phenotype.9 Cichocki et al posit that iDuo NK cells are superior anti-cancer agents to peripheral blood-derived NK cells (PB-NKs). It is interesting that iDuo NK cells upregulate activating receptor expression,1 potentially due to continued IL-15RF stimulation in cis and trans. However, it is worth noting that even without additional modification, ex vivo expanded PB-NKs have consistent and high expression of activating receptors, are functionally mature with robust cytotoxic capacity, and express high levels of KIRs that play an important role in NK cell education and licensing.10 A direct comparison of cell killing between iDuo NK cells and similarly modified PB-NKs (with and without rituximab) is critical to truly ascertain superiority.
Ultimately, Cichocki et al present an exciting approach to targeted cell therapy that can mitigate treatment resistance due to antigen loss or tumor heterogeneity. NK cells are powerful tools with innate cytotoxic mechanisms that can complement the specific cell killing mediated by CARs. The genetic engineering of iDuo NK cells realizes this potential, with the iPSC-derived product having unique advantages for clinical translation. Given the preclinical data, iDuo NK cells are likely to have anti-tumor activity against B-cell malignancies when tested in clinical trial. The engineered iPSC platform is also well suited to parallel translation with CARs to alternate targets for malignancies similarly in need of visionary therapies. Discovery necessarily will continue such that the full potential of NK cells as fully effective anticancer immunotherapeutics can be realized.
Conflict-of-interest disclosure: C.L.B. has pending patent applications describing the use of CAR-NK cells as therapeutics and has received research support from Merck, Sharp, and Dohme, Inc, Bristol-Myers Squibb, and Kiadis, Pharma. R.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal