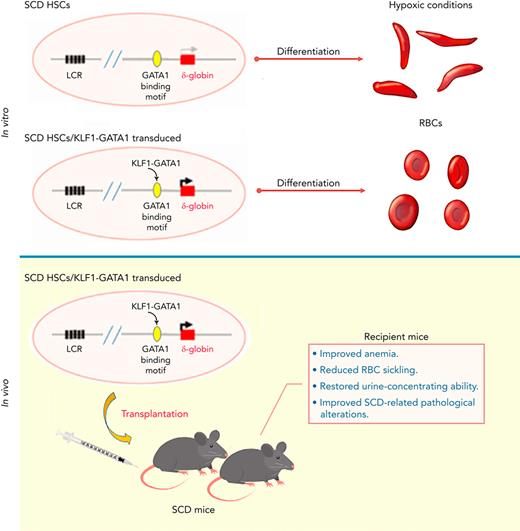
Key Points
KLF1-GATA1 fusion protein enhances δ-globin expression, reducing hypoxia-related sickling in cultured SCD human and murine erythroid cells.
Transplantation of KLF1-GATA1–expressing SCD mouse HSCs into SCD mice improves anemia, RBC sickling, and pathology in recipient mice.
Abstract
Sickle cell disease (SCD) and β-thalassemia are among the most common genetic disorders worldwide, affecting global health and mortality. Hemoglobin A2 (HbA2, α2δ2) is expressed at a low level in adult blood due to the lack of the Kruppel-like factor 1 (KLF1) binding motif in the δ-globin promoter region. However, HbA2 is fully functional as an oxygen transporter, and could be a valid antisickling agent in SCD, as well as a substitute for hemoglobin A in β-thalassemia. We have previously demonstrated that KLF1-GATA1 fusion protein could interact with the δ-globin promoter and increase δ-globin expression in human primary CD34+ cells. We report the effects of 2 KLF1-GATA1 fusion proteins on hemoglobin expression, as well as SCD phenotypic correction in vitro and in vivo. Forced expression of KLF1-GATA1 fusion protein enhanced δ-globin gene and HbA2 expression, as well as reduced hypoxia-related sickling, in erythroid cells cultured from both human sickle CD34+ cells and SCD mouse hematopoietic stem cells (HSCs). The fusion proteins had no impact on erythroid cell differentiation, proliferation, and enucleation. Transplantation of highly purified SCD mouse HSCs expressing KLF1-GATA1 fusion protein into SCD mice lessened the severity of the anemia, reduced the sickling of red blood cells, improved SCD-related pathological alterations in spleen, kidney, and liver, and restored urine-concentrating ability in recipient mice. Taken together, these results indicate that the use of KLF1-GATA1 fusion constructs may represent a new gene therapy approach for hemoglobinopathies.
Introduction
Sickle cell disease (SCD) and β-thalassemia are among the most prevalent and severe monogenetic disorders worldwide, representing a major public health problem.1,2 SCD is caused by a single point mutation in the β-globin gene, leading to production of sickle hemoglobin (HbS, α2βS2). Upon deoxygenation, HbS undergoes polymerization, resulting in erythrocyte deformation, blood flow disruption, and hemolysis, thus affecting multiple organs and causing severe clinical complications.3
The primary treatment for SCD has been focused on the use of hydroxyurea (HU), to increase fetal hemoglobin (HbF) production (which then inhibits Hb polymerization), and on providing supportive care.4,5 However, HU is not curative and does not appear to reverse established end-organ damage. At present, the only curative treatment for SCD is allogeneic hematopoietic stem cell (HSC) transplantation,6,7 but this approach is limited by donor availability.8,9 Autologous HSC transplantation provides patients with SCD with alternative curative therapies.10 Current gene therapy approaches for SCD include βS-globin gene correction, induction of HbF, and globin gene addition.11,12 βS-Globin gene correction and induction of HbF can be achieved by the CRISPR-Cas9 genome-editing technology; however, this cutting-edge technology is still premature and has severe limitations and considerations.13,14 On the other hand, addition of a globin gene can be achieved by lentivirus-based HSC transplantation, which significantly addresses the safety and efficacy issues and has displayed a promising clinical outcome for hemoglobinopathies.15,16
Both HbA2 (HbA2, α2δ2) and HbF (α2γ2) are minor components in adult blood17,18 and have been shown to be equally effective in inhibiting intracellular deoxy-HbS polymerization.19,20 Kruppel-like factor 1 (KLF1) and GATA1 are transcriptional factors essential for erythroid cell proliferation, differentiation, and Hb production.21-25 The low expression of δ-globin in adult blood is related to the lack of the KLF1-binding site (CACCC box) within the δ-globin proximal promoter region. Insertion of this binding site activates δ-globin promoter activity in erythroid cells26-28 and increases HbA2 levels, with a concomitant phenotype improvement in β-thalassemic mice.29 The transactivation domain of KLF1 serves as a positive regulator,28 and the GATA1-binding motif in the δ-globin proximal promoter is a functional regulator for δ-globin expression.30,31 Therefore, a KLF1 fusion protein containing its transactivation domains and the GATA1-binding motif should positively regulate δ-globin production. Our prior study has demonstrated that KLF1-GATA1 fusion proteins composed of KLF1 transactivation domains and GATA1 DNA-binding domains could interact with the δ-globin promoter and increase δ-globin expression in human CD34+ cells.32 A recent study has shown that δ-globin gene expression improves the SCD phenotype in a humanized mouse model.33 These findings led us to believe that human δ-globin gene activation could represent an alternative genetic therapeutic approach to hemoglobinopathies.
This study was conducted to further characterize the capacity of KLF1-GATA1 fusion proteins to induce δ-globin and to correct the sickling phenotype in vitro and in vivo. We found that forced expression of medium- and long-form KLF1-GATA1 fusion proteins significantly increases δ-globin expression and reduces hypoxia-related sickling in SCD human and mouse erythroid cells in vitro. Our in vivo studies demonstrated that lentivirus-based transplantation of KLF1-GATA1–expressing HSCs into SCD mice lessens the severity of anemia, reduces the sickling of red blood cells, improves SCD-related pathological alterations in spleen, kidney, and liver, and restores urine-concentrating ability in recipient mice. These results strongly suggest that such fusion constructs could be a valuable genetic therapeutic tool for hemoglobinopathies.
Materials and methods
Primary human sickle CD34+ cell preparation and culture
Human red blood samples discharged from patients with SCD red blood exchange were acquired according to guidelines approved by the National Institutes of Health. These samples were exempt from Institutional Review Board review, as they are deemed anonymous medical waste. Mononuclear cells were isolated from blood samples using Ficoll Hypaque (GE Healthcare, Chicago, IL). CD34+ cells were enriched using the StemSep Human CD34 Positive Selection Cocktail (Stemcell Technologies, Seattle, WA) per the manufacturer’s instructions. An in vitro model for supporting erythroid-directed differentiation from human CD34+ cells was used. To support erythroid-directed differentiation, purified human CD34+ cells isolated from blood samples from individuals with SCD (human sickle CD34+ cells) were cultured in a 3-week serum-free system consisting of 3 phases (phase 1 from day 0 to 7, phase 2 from day 7 to 14, and phase 3 from day 14 to 21), as previously described.34
Mouse sickle HSC (Sca-1+c-Kit+Lin/low) isolation and culture
Berkeley SCD mice, which have been engineered for HbS production,35 were purchased from The Jackson Laboratory (Sacramento, CA) and maintained according to the company’s instructions. The Animal Care and Use Committee of the National Heart, Lung, and Blood Institute approved the experiments. Bone marrow from femurs and tibia was isolated from 8 to 20-week-old SCD mice, according to a published method.36 After the red blood cells were lysed (ACK Lysing Buffer; Lonza, Walkersville, MD), Sca-1+c-Kit+Lin/low cells were collected by fluorescence-activated cell sorting (using lineage-specific antibodies and Sca-1 and c-kit antibodies from Miltenyi Biotec, Gaithersburg, MD) on a FACSVantage SE (BD Biosciences, San Jose, CA) flow cytometer. The sorted cells (SCD mouse HSCs) were cultured in a 3-week serum-free system consisting of 3 phases. During phase 1 of culture (days 0-7), mouse HSCs were incubated in StemSpan medium (Stemcell Technologies) supplemented with 10 ng/mL mouse stem cell factor (Miltenyi Biotec), 50 ng/mL mouse thrombopoietin (Miltenyi Biotec), 20 ng/mL mouse insulinlike growth factor 2 (R&D Systems, Minneapolis, MN), 10 ng/mL human fibroblast growth factor 1 (R&D Systems), 3 IU/mL heparin (Sigma-Aldrich, St. Louis, MO), 50 IU/mL penicillin and streptomycin, and 2 mmol/L glutamine. During phase 2 (days 7-14), cells were grown in StemPro-34 complete medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 4 IU/mL erythropoietin (Amgen, Thousand Oaks, CA), 20 ng/mL stem cell factor, 10 μg/mL insulin (Sigma-Aldrich), 3 IU/mL heparin, and 0.8 mg/mL holo-transferrin (Sigma-Aldrich). On culture day 14, the cells were counted and transferred to phase 3 medium at 8 × 105 cells/mL for the remaining 7 days of culture. Phase 3 medium was StemPro-34 complete medium supplemented with 4 IU/mL erythropoietin, 3 μM RU486 (Sigma-Aldrich), 10 μg/mL insulin, 3 IU/mL heparin, and 0.8 mg/mL holo-transferrin.
Plasmid construction and lentivirus production
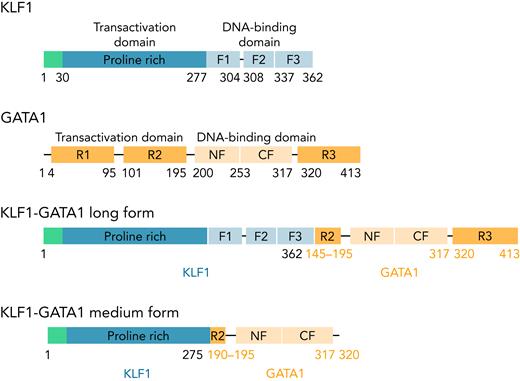
KLF1, GATA1, and 2 fusion pLenti V5 KLF1-GATA1 expression vectors (Figure 1) were constructed, and a lentivirus encoding KLF1, GATA1, KLF1-GATA1, or vector only was packaged and concentrated, as previously reported.37
The structure of KLF1, GATA1, and KLF1-GATA1 fusion constructs. F1, F2, and F3 represent 3 finger domains of KLF1; CF and NF represent the C- and N-fingers of GATA1; R1, R2, and R3 represent 3 regions of the transactivation domain of GATA1.
The structure of KLF1, GATA1, and KLF1-GATA1 fusion constructs. F1, F2, and F3 represent 3 finger domains of KLF1; CF and NF represent the C- and N-fingers of GATA1; R1, R2, and R3 represent 3 regions of the transactivation domain of GATA1.
Low oxygen exposure and assessment of cellular morphologies
For studies of hypoxia-related sickling, during the final week in culture, cells cultured from human CD34+ sickle cells or SCD mouse HSCs were incubated in a 2% oxygen environment consistent with the lower oxygen range estimated for human bone marrow.38
For cells cultured from human CD34+ sickle cells, cellular morphologies were assessed according to published methods.39 Cell imaging was accomplished with an inverted microscope (32× magnification) equipped with a AxioCam MRc5 camera (Carl Zeiss, Oberkochen, Germany). Four random fields were imaged from each sample, and cellular morphologies were scored by blinded observers.
Cells cultured from SCD mouse HSCs were subjected to sickle imaging flow cytometry assays.40,41 In brief, cells incubated at 2% oxygen were fixed with glutaraldehyde (final concentration, 2.5%; Sigma-Aldrich), washed, and stained with TER119-APC antibody (Miltenyi Biotec) and thiazole orange (TO) dye (Sigma-Aldrich) before the samples were analyzed in an ImageStream X imaging flow cytometer (Amnis Corporation, Seattle, WA). Data were acquired using Inspire acquisition software (Atlanta, GA) and a 60× objective lens. Data from a minimum of 10 000 cells were collected for each sample and analyzed with Ideas 5.0 software (Amnis Corporation).
Transplantation of SCD mouse HSCs
Newly sorted Sca-1+c-Kit+Lin/low cells from SCD mice were directly incubated with lentivirus without prestimulation overnight. Viral particles (1.2 × 108) were preloaded onto RetroNectin-coated plates (Takara Bio, San Jose, CA) and incubated at 37°C for 4 to 5 hours. The viral supernatant was discarded just before infection, Sca-1+c-Kit+Lin/low cells (4 × 106) in phase 1 culture medium were loaded onto plates, and cells were returned to the incubator. The following day, 3 × 106 cells were injected by tail vein into SCD mouse recipients (8-12 weeks old) that had received 400 cGy of total body irradiation. Transplant-recipient mice were maintained under specific pathogen-free conditions, with antibiotics (Baytril, 4 mL/350 mL water; Bayer Healthcare LLC; Whippany, NJ) added to the drinking water for 2 weeks after transplantation. Recipient animals were immunosuppressed the day before and for 2 weeks after bone marrow cell transplantation by intraperitoneal injection of Rapamune (NedChemExpress, Monmouth Junction, NJ).
Hematologic analysis, reticulocyte analysis, and organ pathology
Blood samples were collected from the lateral saphenous vein with a 23-gauge needle 20 to 24 weeks after transplantation. An automated blood cell analyzer (Hemavet 3700; CDC Technologies, Oxford, CT) was used to obtain complete blood counts. Reticulocyte analysis was performed according to a published method.42 At necropsy, mice were euthanized and spleen weights recorded. Tissues collected for microscopic evaluation were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with either hematoxylin and eosin to identify architecture or with Prussian blue to evaluate iron deposition.
Urine-concentrating ability
Mice were deprived of food and water for 12 to 14 hours. At the end of this period, urine was collected onto Parafilm, and the osmolarity measured with a Vapor Pressure Osmometer (ELITech Group, Logan, UT).
Results
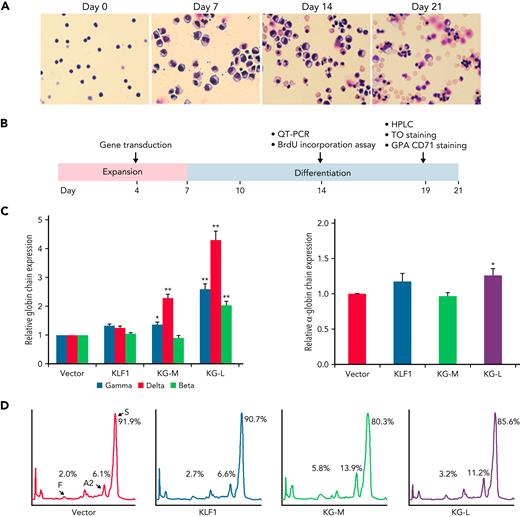
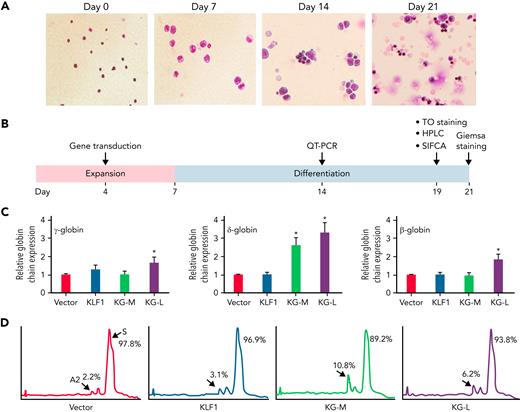
KLF1-GATA1 fusion protein upregulates δ-globin gene expression in erythroid cells cultured from human sickle CD34+ cells
We have previously characterized a series of KLF1-GATA1 fusion proteins composed of different combinations of KLF1 transactivation domains and GATA1 DNA-binding domains and have demonstrated that 2 of these fusion proteins could interact with the δ-globin promoter and increase δ-globin expression in primary CD34+ cells.32 To explore whether these fusion constructs could be a valuable genetic therapeutic tool for hemoglobinopathies, we overexpressed KLF1-GATA1 in CD34+ cells isolated from patients with SCD by using lentiviruses expressing KLF1-GATA1 fusion protein, cultured the transduced cells to the mature stage of erythroid (Figure 2A), and evaluated the effects of KLF1-GATA1 on Hb expression and sickling phenotype correction (Figure 2B). The long-form KLF1-GATA1 upregulated mean β-globin gene expression 2.0-fold, mean δ-globin gene expression 4.3-fold, and mean γ-globin gene expression 2.6-fold; the medium-form KLF1-GATA1 upregulated mean δ-globin gene expression 2.3-fold and γ-globin gene expression 1.3-fold, but had no significant effect on mean β-globin gene expression (Figure 2C, left). Further, transduction of KLF1-GATA1 fusion proteins increased HbA2+HbF expression from 8.1% ± 2.9% in vector-transduced cells to 19.7% ± 3.7% in medium-form KLF1-GATA1–transduced cells (P < .01) and 14.4% ± 3.2% in long-form KLF1-GATA1–transduced cells (P < .01). HbS expression was reduced from 91.9% ± 2.9% in vector-transduced cells to 80.3% ± 3.7% in medium-form KLF1-GATA1–transduced cells (P < .01) and 85.7% ± 3.2% in long-form KLF1-GATA1–transduced cells (Figure 2D; P < .01). Although the KLF1-GATA1 fusion proteins substantially increased the transcription of β-like globin genes, the long-form KLF1-GATA1 upregulated α-globin gene expression only very moderately (by a factor of 1.2-fold), and the medium-form KLF1-GATA1 had no impact on α-globin gene expression (Figure 2C, right). It has been reported that the effect of a single α- or β-thalassemia allele can be completely silent with no definable hematological abnormalities.43,44 Therefore, it is unlikely that the upregulation of β-like globin gene expression (which produces globin chain imbalance) by the fusion proteins would cause a thalassemialike phenotype.
KLF1-GATA1 fusion protein upregulates δ-globin gene and HbA2 expression in erythroid cells cultured from human CD34+ sickle cells. Human CD34+ sickle cells were lentivirus transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in an erythroid culture system for 3 weeks. (A) Cultured cells stained with Giemsa showing erythroid differentiation progression from erythroblast to normoblast at days 7 and 14 to a mostly uniform population of enucleated reticulocytes and erythrocytes at day 21. Original magnification, ×40. (B) Experimental schema, with the time course of expansion and differentiation phases indicated. Time points are indicated for the specific analyses (C) Quantitative-PCR (QT-PCR) analysis of globin-chain gene expression in cultured erythroid cells. ∗P < .05, ∗∗P < .01 vs vector control-transduced cells. Error bars represent the mean ± standard deviation (SD) of 3 independent experiments. (D) HPLC analysis of HbA, HbA2, and HbF expression in cultured erythroid cells. At least 3 independent experiments were performed. HPLC, high performance liquid chromatography; KG-M, medium-form KLF1-GATA1; KG-L, long-form KLF1-GATA1.
KLF1-GATA1 fusion protein upregulates δ-globin gene and HbA2 expression in erythroid cells cultured from human CD34+ sickle cells. Human CD34+ sickle cells were lentivirus transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in an erythroid culture system for 3 weeks. (A) Cultured cells stained with Giemsa showing erythroid differentiation progression from erythroblast to normoblast at days 7 and 14 to a mostly uniform population of enucleated reticulocytes and erythrocytes at day 21. Original magnification, ×40. (B) Experimental schema, with the time course of expansion and differentiation phases indicated. Time points are indicated for the specific analyses (C) Quantitative-PCR (QT-PCR) analysis of globin-chain gene expression in cultured erythroid cells. ∗P < .05, ∗∗P < .01 vs vector control-transduced cells. Error bars represent the mean ± standard deviation (SD) of 3 independent experiments. (D) HPLC analysis of HbA, HbA2, and HbF expression in cultured erythroid cells. At least 3 independent experiments were performed. HPLC, high performance liquid chromatography; KG-M, medium-form KLF1-GATA1; KG-L, long-form KLF1-GATA1.
KLF1-GATA1 fusion protein reduces hypoxia-related sickling without effect on differentiation and proliferation in erythroid cells cultured from human sickle CD34+ cells
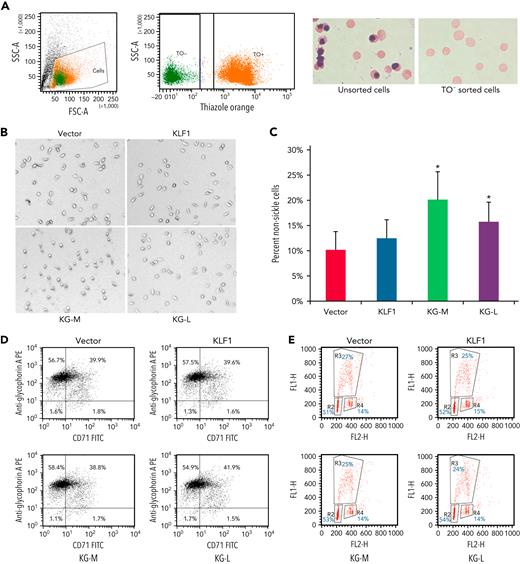
To evaluate the effect of KLF1-GATA1 fusion proteins on sickle phenotype correction, erythroid cells cultured from human sickle CD34+ cells and exposed to low-oxygen conditions were stained with TO, then subjected to cell sorting. TO− cells (Figure 3A; enucleated cells) were sorted directly into wells containing culture medium and incubated for an additional 16 hours at 2% oxygen before imaging studies. Upon deoxygenation, the mean percentage of nonsickling erythroid cells significantly increased to 20.1% ± 5.4% and 15.1% ± 3.9% in medium- and long-form KLF1-GATA1–transduced cells, respectively, as compared with 10.2% ± 3.5% in vector-transduced cells (Figure 3B-C). Transduction with KLF1 had no significant effect on erythroid cell δ-globin gene expression, HbA2+HbF expression, or sickling morphology (Figures 2C-D and 3B-C). Our results suggest that, although the long-form and medium-form KLF1-GATA1 fusion proteins had different effects on inducing individual globin genes, both forms significantly reduced sickling morphology in erythroid cells cultured from human CD34+ sickle cells subjected to hypoxic conditions. Flow-cytometric analyses of transferrin receptor/glycophorin A expression and bromodeoxyuridine incorporation assays for erythroid cells cultured from human sickle CD34+ cells showed that both erythroid cell differentiation and proliferation were not affected by transduction with KLF1-GATA1 fusion protein (Figure 3D-E).
KLF1-GATA1 fusion protein reduces hypoxia-related sickling in erythroid cells cultured from CD34+ cells isolated from blood samples of patients with SCD. (A) CD34+ cells isolated from patients with SCD were lentivirus-transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in a 3-phase erythroid culture system.39 (A) At day 19, cultured erythroid cells were stained with TO for sorting enucleated erythroid cells. Unsorted or TO− sorted cells (enucleated erythroid cells) were cytospun, followed by Giemsa staining. (B) Representative bright-field images of enucleated erythroid cells that were incubated for another 16 hours in a 2% oxygen environment in culture medium before image processing. All images were taken within 3 minutes after removal from 2% oxygen without further manipulating the cells. Original magnification ×35. (C) Mean percentage of nonsickled (round) erythroid cells after a 16-hour incubation in a 2% oxygen environment. ∗P < .05 vs vector control-transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (D) Flow cytometry analysis of cultured erythroid cells for erythroid differentiation markers glycophorin A and transferrin receptor (CD71). At least 3 independent experiments were performed. (E) Bromodeoxyuridine incorporation assay of cultured erythroid cells for cell proliferation. R2, R3, and R4 represent the G1, S, and G2 phases, respectively. At least 3 independent experiments were performed. KG-M, KLF1-GATA1 medium form; KG-L, KLF1-GATA1 long form.
KLF1-GATA1 fusion protein reduces hypoxia-related sickling in erythroid cells cultured from CD34+ cells isolated from blood samples of patients with SCD. (A) CD34+ cells isolated from patients with SCD were lentivirus-transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in a 3-phase erythroid culture system.39 (A) At day 19, cultured erythroid cells were stained with TO for sorting enucleated erythroid cells. Unsorted or TO− sorted cells (enucleated erythroid cells) were cytospun, followed by Giemsa staining. (B) Representative bright-field images of enucleated erythroid cells that were incubated for another 16 hours in a 2% oxygen environment in culture medium before image processing. All images were taken within 3 minutes after removal from 2% oxygen without further manipulating the cells. Original magnification ×35. (C) Mean percentage of nonsickled (round) erythroid cells after a 16-hour incubation in a 2% oxygen environment. ∗P < .05 vs vector control-transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (D) Flow cytometry analysis of cultured erythroid cells for erythroid differentiation markers glycophorin A and transferrin receptor (CD71). At least 3 independent experiments were performed. (E) Bromodeoxyuridine incorporation assay of cultured erythroid cells for cell proliferation. R2, R3, and R4 represent the G1, S, and G2 phases, respectively. At least 3 independent experiments were performed. KG-M, KLF1-GATA1 medium form; KG-L, KLF1-GATA1 long form.
KLF1-GATA1 fusion protein upregulates δ-globin gene and HbA2 expression and reduces hypoxia-related sickling in erythroid cells cultured from SCD mouse HSCs
To investigate whether the effect of KLF1-GATA1 fusion protein on Hb expression and sickle phenotype in erythroid cells cultured from human CD34+ sickle cells could be extrapolated to mouse lineage-negative stem cells, we transduced Sca-1+c-Kit+Lin/low HSCs isolated from the bone marrow of SCD mice with lentiviral particles encoding KLF1-GATA1 fusion protein, then cultured to differentiate into mature erythroid cells (Figure 4A) and evaluated (Figure 4B). Quantitative polymerase chain reaction analysis showed that both long-form and medium-form KLF1-GATA1 fusion proteins enhanced globin gene expression compared with vector control (Figure 4C). Long-form KLF1-GATA1 upregulated mean gene expression of β-, δ-, and γ-globin by 1.8-fold, 3.3-fold, and 1.7-fold, respectively. Medium-form KLF1-GATA1 upregulated mean δ-globin gene expression 2.6-fold, but had no significant effect on β-globin expression. Transduction of KLF1-GATA1 fusion proteins increased HbA2 expression from 2.1% ± 0.5% in vector-transduced cells to 8.9% ± 1.9% in medium-form KLF1-GATA1–transduced cells (P < .01) and 6.3% ± 1.4% in long-form KLF1-GATA1–transduced cells (Figure 4D; P < .01). Transduction with KLF1 did not affect δ-globin gene or HbA2 expression.
KLF1-GATA1 fusion protein upregulates δ-globin gene and HbA2 expression in erythroid cells cultured from SCD mouse HSCs. Sca-1+c-Kit+Lin/low HSCs isolated from SCD mice were transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in an erythroid culture system for 3 weeks. (A) Giemsa staining at the cell-culture time points indicated, showing the progression of erythroid differentiation from HSCs to enucleated reticulocytes and erythrocytes. Original magnification, ×40. (B) The time course of expansion and differentiation phases. Time points are indicated for the specific analyses conducted. (C) Quantitative-PCR (QT-PCR) analysis of globin-chain gene expression in cultured erythroid cells. ∗P < .05, ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (D) Representative HPLC analysis of HbA2 expression in cultured erythroid cells. At least 3 independent experiments were performed. HPLC, high performance liquid chromatography; KG-M, medium-form KLF1-GATA1; KG-L, long-form KLF1-GATA1.
KLF1-GATA1 fusion protein upregulates δ-globin gene and HbA2 expression in erythroid cells cultured from SCD mouse HSCs. Sca-1+c-Kit+Lin/low HSCs isolated from SCD mice were transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in an erythroid culture system for 3 weeks. (A) Giemsa staining at the cell-culture time points indicated, showing the progression of erythroid differentiation from HSCs to enucleated reticulocytes and erythrocytes. Original magnification, ×40. (B) The time course of expansion and differentiation phases. Time points are indicated for the specific analyses conducted. (C) Quantitative-PCR (QT-PCR) analysis of globin-chain gene expression in cultured erythroid cells. ∗P < .05, ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (D) Representative HPLC analysis of HbA2 expression in cultured erythroid cells. At least 3 independent experiments were performed. HPLC, high performance liquid chromatography; KG-M, medium-form KLF1-GATA1; KG-L, long-form KLF1-GATA1.
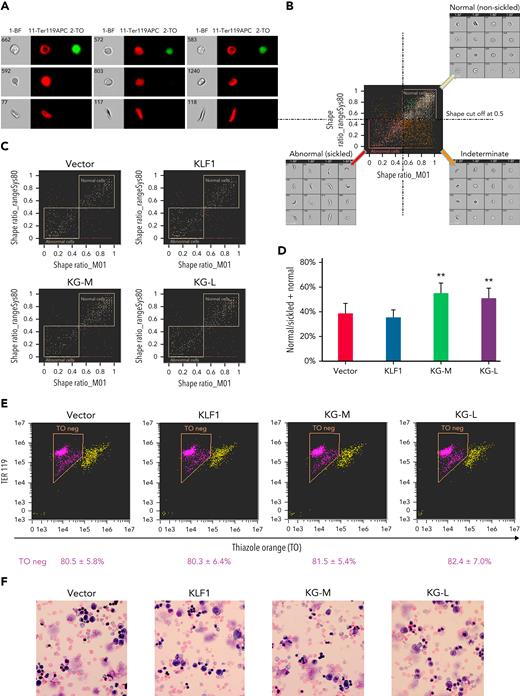
To evaluate the effect of KLF1-GATA1 fusion proteins on erythroid cell sickling under hypoxic conditions, KLF1- or KLF1-GATA1–transduced SCD mouse HSCs were incubated in a 2% oxygen environment during the third week in culture, then subjected to sickle imaging flow cytometry assays (Figures 5A-B). Our data revealed that KLF1-GATA1 increased the mean percentage of hypoxia-related nonsickling erythroid cells from 38.1% ± 8.0% in vector-transduced cells to 57.2% ± 8.0% in medium-form KLF1-GATA1–transduced cells and to 50.7% ± 7.6% in long-form KLF1-GATA1–transduced cells. Transduction with KLF1 had no impact on erythroid cell sickling induced by hypoxia (Figures 5C-D).
KLF1-GATA1 fusion protein reduces hypoxia-related sickling in erythroid cells cultured from SCD mouse HSCs. Sca-1+c-Kit+Lin/low HSCs isolated from SCD mice were transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in a 3-phase erythroid culture system. (A–D) SIFCA of cultured erythroid cells stained with TER119-APC antibody and TO. (A) Bright-field and fluorescence images of erythroid cells acquired by imaging flow cytometry, illustrating examples of the cell morphologies. (B) SIFCA identifying the shape ratio cutoff of 0.5 used for classifying cultured erythroid cells as normal/round, abnormal/sickled, or undefined/indeterminate, and associated representative bright-field imagery. Shape ratio is the minimum width of the cell divided by the maximum length identified by the analysis software. Cells with a shape ratio lower than the cutoff values were designated as “abnormal cells (sickled cells).” Cells with a shape ratio higher than the cutoff level were defined as “normal cells (non-sickled cells).” Cells were designated as “undefined” if the cells could not be categorized.87,88 (C) Representative SIFCA of cultured erythroid cells. (D) Graphic representation showing the mean percentage of normal cells identified through SIFCA. ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD of 3 independent experiments. (E) Representative flow-cytometric analysis of cultured erythroid cells stained with TER119-APC antibody and TO to assess erythroid enucleation. Data represent the mean ± SD of 3 independent experiments. (F) Giemsa staining of cultured erythroid cells. Original magnification, ×40. All analyses were performed at the time points indicated in Figure 4B. KG-M, KLF1-GATA1 medium-form; KG-L, KLF1-GATA1 long-form; SIFCA, sickle cell imaging flow cytometry assay.
KLF1-GATA1 fusion protein reduces hypoxia-related sickling in erythroid cells cultured from SCD mouse HSCs. Sca-1+c-Kit+Lin/low HSCs isolated from SCD mice were transduced with vector only, KLF1, or KLF1-GATA1 fusion protein, then grown in a 3-phase erythroid culture system. (A–D) SIFCA of cultured erythroid cells stained with TER119-APC antibody and TO. (A) Bright-field and fluorescence images of erythroid cells acquired by imaging flow cytometry, illustrating examples of the cell morphologies. (B) SIFCA identifying the shape ratio cutoff of 0.5 used for classifying cultured erythroid cells as normal/round, abnormal/sickled, or undefined/indeterminate, and associated representative bright-field imagery. Shape ratio is the minimum width of the cell divided by the maximum length identified by the analysis software. Cells with a shape ratio lower than the cutoff values were designated as “abnormal cells (sickled cells).” Cells with a shape ratio higher than the cutoff level were defined as “normal cells (non-sickled cells).” Cells were designated as “undefined” if the cells could not be categorized.87,88 (C) Representative SIFCA of cultured erythroid cells. (D) Graphic representation showing the mean percentage of normal cells identified through SIFCA. ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD of 3 independent experiments. (E) Representative flow-cytometric analysis of cultured erythroid cells stained with TER119-APC antibody and TO to assess erythroid enucleation. Data represent the mean ± SD of 3 independent experiments. (F) Giemsa staining of cultured erythroid cells. Original magnification, ×40. All analyses were performed at the time points indicated in Figure 4B. KG-M, KLF1-GATA1 medium-form; KG-L, KLF1-GATA1 long-form; SIFCA, sickle cell imaging flow cytometry assay.
Flow-cytometric analyses of TER119/TO staining indicated that erythroid cell enucleation was not affected by KLF1-GATA1 transduction (Figure 5E). Similarly, Giemsa staining showed a mostly uniform population of enucleated reticulocytes and erythrocytes in all the groups without a notable morphology difference (Figure 5F).
Therapeutic use of KLF1-GATA1 fusion protein improves the SCD phenotype in vivo
We next determined the effect of KLF1-GATA1 fusion protein on pathological changes in SCD-related organs. Mock- or KLF1-GATA1–transduced SCD mouse HSCs were transplanted into SCD mice, then peripheral blood, spleen, kidney, and liver tissues were collected at 20 to 24 weeks after transplantation for evaluation. Compared with mock mice (SCD mouse recipients of mock-transduced SCD mouse HSCs), the SCD mice receiving KLF1-GATA1–transduced SCD mouse HSCs (KG-M mice received medium form–transduced SCD mouse HSCs] and KG-L mice received long form–transduced SCD mouse HSCs), collectively referred as to KG mice, exhibited a marked increase in red blood cell counts, Hb levels, and hematocrit values and significantly reduced reticulocytosis (Table 1). The mean percentage of HbA2 in peripheral blood was increased, and the percentage of sickling cells was reduced in KG mice compared with mock mice (Table 1; Figure 6A). KG-M mice demonstrated a greater improvement in all these parameters compared with KG-L mice. Reduced SCD-related pathology was observed in spleen, kidney, and liver tissues of KG mice compared with mock mice (Figures 6B-D). The spleens of mock-transduced mice displayed excessive extramedullary hematopoiesis in the red pulp, with a dramatic loss of lymphoid follicular structure. Although these abnormalities were still present in the KG-L mice, the severity was markedly decreased. KG-M mice displayed a relatively normal splenic architecture (splenic red and white pulps observed), with mildly increased erythropoiesis (Figure 6B). In addition, splenomegaly was substantially reduced in KG mice compared with mock mice (Figure 6E; Table 1). The kidneys of mock mice displayed congestion and dilation of medullary capillaries, with sickled red blood cells clearly present (Figure 6C, top row). Those mice also exhibited impaired urine-concentrating ability (Table 1), most likely because of reduced medullary blood flow that caused extensive tubular damage. Further, their kidneys accumulated abundant iron deposition in the proximal renal tubules (Figure 6C, bottom 2 rows). In contrast, the SCD-related pathology of the kidneys in KG mice was substantially improved. The kidneys of KG-L mice did not exhibit the disruptive vascular congestion and iron deposits that were observed in mock mice; KG-M mice showed mildly congested vasculature and reduced iron deposition in kidneys (Figure 6C). Moreover, urine-concentrating ability was increased in KG mice compared with mock mice (Table 1). Last, iron deposition was remarkably reduced in the liver of KG mice compared with that in mock mice (Figure 6D).
Analysis of biological samples collected from SCD mice after transplantation of KLF1-GATA1–expressing SCD mouse HSCs
| Transplant group . | n . | Peripheral blood . | Spleen . | Urine . | |||||
|---|---|---|---|---|---|---|---|---|---|
| RBCs, ×106/μL . | Hb, g/dL . | WBCs, ×103/μL . | Hematocrit, % . | HbA2, % . | Reticulocytes, % . | Weight, g . | Osmolarity, mOsm/kg . | ||
| Mock mice | 11 | 5.73 ± 1.24 | 6.48 ± 1.45 | 14.89 ± 6.45 | 26.25 ± 7.56 | 4.14 ± 1.55 | 50.47 ± 6.33 | 1.14 ± 0.25 | 1462 ± 236 |
| KG-M mice | 11 | 9.86 ± 1.71∗∗ | 11.61 ± 2.93∗∗ | 9.86 ± 4.80∗ | 39.34 ± 5.97∗∗ | 18.81 ± 3.85∗∗ | 21.45 ± 7.16∗∗ | 0.75 ± 0.10∗∗ | 2663 ± 305∗∗ |
| KG-L mice | 10 | 7.16 ± 0.62∗∗ | 7.84 ± 1.00∗ | 15.71 ± 6.14 | 31.96 ± 3.34∗ | 11.47 ± 1.11∗∗ | 32.56 ± 7.41∗∗ | 0.90 ± 0.20∗ | 1985 ± 326∗∗ |
| Transplant group . | n . | Peripheral blood . | Spleen . | Urine . | |||||
|---|---|---|---|---|---|---|---|---|---|
| RBCs, ×106/μL . | Hb, g/dL . | WBCs, ×103/μL . | Hematocrit, % . | HbA2, % . | Reticulocytes, % . | Weight, g . | Osmolarity, mOsm/kg . | ||
| Mock mice | 11 | 5.73 ± 1.24 | 6.48 ± 1.45 | 14.89 ± 6.45 | 26.25 ± 7.56 | 4.14 ± 1.55 | 50.47 ± 6.33 | 1.14 ± 0.25 | 1462 ± 236 |
| KG-M mice | 11 | 9.86 ± 1.71∗∗ | 11.61 ± 2.93∗∗ | 9.86 ± 4.80∗ | 39.34 ± 5.97∗∗ | 18.81 ± 3.85∗∗ | 21.45 ± 7.16∗∗ | 0.75 ± 0.10∗∗ | 2663 ± 305∗∗ |
| KG-L mice | 10 | 7.16 ± 0.62∗∗ | 7.84 ± 1.00∗ | 15.71 ± 6.14 | 31.96 ± 3.34∗ | 11.47 ± 1.11∗∗ | 32.56 ± 7.41∗∗ | 0.90 ± 0.20∗ | 1985 ± 326∗∗ |
Data are presented as means ± standard deviation.
Hb, total Hb concentration; RBCs, red blood cells; WBCs, white blood cells.
P < .05; ∗∗P < 0.01 vs mock mice.
Transplantation of KLF1-GATA1–expressing SCD mouse HSCs into SCD mice reduces the percent of sickling cells in peripheral blood and improves SCD-related pathologic changes in spleen, kidney, and liver. Mock- or KLF1-GATA1–transduced SCD mouse HSCs were transplanted into SCD mice. Recipient mice were euthanized 20 to 24 weeks after transplantation, and peripheral blood and tissues were collected. Tissue sections were stained to evaluate organ pathology and iron accumulation. (A) Representative Giemsa staining of peripheral blood smear for evaluating erythrocyte morphology. Bar represents 20 μm. Images were acquired with an Olympus BX51 microscope (Olympus, Center Valley, PA) and a Qimaging Camera with Q Capture pro software (Qimaging, Surrey, BC, Canada). Four random fields were imaged from each sample, and cellular morphologies were scored by blinded observers. ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (B) Representative spleen tissue stained with hematoxylin and eosin (H&E). Bars represent 50 μm. (C) Top row: representative kidney tissue stained with H&E. Bar represents 50 μm. Middle and bottom rows: representative kidney tissue stained with Prussian blue for iron content. Bar represents 100 μm (middle) and 20 μm (bottom). (D) Representative liver sections stained with Prussian blue for iron content. Bar represents 50 μm. (E) Representative spleens collected from recipient mice. Mock mice are SCD mice with mock-transduced SCD mouse HSC transplants; KG-M and KG-L mice are SCD mice that receive transplants of medium- and long-form KLF1-GATA1–transduced SCD mouse HSCs, respectively.
Transplantation of KLF1-GATA1–expressing SCD mouse HSCs into SCD mice reduces the percent of sickling cells in peripheral blood and improves SCD-related pathologic changes in spleen, kidney, and liver. Mock- or KLF1-GATA1–transduced SCD mouse HSCs were transplanted into SCD mice. Recipient mice were euthanized 20 to 24 weeks after transplantation, and peripheral blood and tissues were collected. Tissue sections were stained to evaluate organ pathology and iron accumulation. (A) Representative Giemsa staining of peripheral blood smear for evaluating erythrocyte morphology. Bar represents 20 μm. Images were acquired with an Olympus BX51 microscope (Olympus, Center Valley, PA) and a Qimaging Camera with Q Capture pro software (Qimaging, Surrey, BC, Canada). Four random fields were imaged from each sample, and cellular morphologies were scored by blinded observers. ∗∗P < .01 vs vector control–transduced cells. Error bars indicate the mean ± SD results of 3 independent experiments. (B) Representative spleen tissue stained with hematoxylin and eosin (H&E). Bars represent 50 μm. (C) Top row: representative kidney tissue stained with H&E. Bar represents 50 μm. Middle and bottom rows: representative kidney tissue stained with Prussian blue for iron content. Bar represents 100 μm (middle) and 20 μm (bottom). (D) Representative liver sections stained with Prussian blue for iron content. Bar represents 50 μm. (E) Representative spleens collected from recipient mice. Mock mice are SCD mice with mock-transduced SCD mouse HSC transplants; KG-M and KG-L mice are SCD mice that receive transplants of medium- and long-form KLF1-GATA1–transduced SCD mouse HSCs, respectively.
Discussion
HU was the first of the now 4 US Food and Drug Administration–approved disease-modifying therapies for SCD; other more effective or curative treatment options are urgently being sought. Gene therapy has long been proposed as a potential cure for SCD.45 Ten SCD gene therapy clinical trials, using different gene-modification techniques, are currently being conducted.46 Most of these trials are in the early phases, with short follow-up times and small patient samples.47,48 One of the major approaches involves viral transduction of HSCs with antisickling globin gene(s). Although this approach is still in the experimental stage, the outcomes from the multicenter clinical trials are very encouraging.49-51 In this study, rather than delivering antisickling globin gene(s) into SCD HSCs, we transduced the KLF1-GATA1 fusion protein that can interact with the δ-globin promoter into SCD HSCs. We demonstrated that forcible expression of KLF1-GATA1 fusion protein in HSCs from SCD mice and patients could significantly increase δ-globin expression and reduce cell sickling in an erythroid culture system. We also found that transplantation of purified SCD mouse HSCs overexpressing KLF1-GATA1 into SCD mice reduced the severity of their anemia, red blood cell sickling, and SCD-related organ pathology. It is noteworthy that current clinical trials of SCD gene therapy still require myeloablative conditioning so that the patient’s own marrow does not reject the genetically modified stem cells.52,53 However, adult patients with severe SCD who exhibit preexisting multiorgan disease cannot tolerate the toxicity of myeloablative conditioning.54 In the current study, in the absence of myeloablative conditioning and cytokine prestimulation, transduction of SCD mouse HSCs with the medium-form KLF1-GATA1 fusion protein produced an appreciable SCD-related phenotype correction in our animal model and may represent an alternative gene therapy treatment for patients with SCD not suited for myeloablation-based gene therapy.
Both HbF (≤2% of total Hb) and HbA2 (2% to 3% of total Hb) are expressed at a low level in normal adult blood. The Cooperative Study of Sickle Cell Disease has provided convincing evidence that increases in HbF levels could result in partial amelioration of clinical severity and mortality and that an elevation of 4% HbF should have clinically significant effects in this disease.55,56 Recent studies have also shown that a small decrease in the intracellular HbS concentration is of therapeutic potential for SCD.57,58 In our study, when KLF1-GATA1–transduced SCD mouse HSCs were transplanted into SCD mice, the percentage of HbA2 and Hb concentration were significantly increased. The degree of elevation in HbA2 (from 4.14% to 18.81% in KG-M mice and from 4.14% to 11.47% in KG-L mice) lowered intracellular HbS concentration and could inhibit HbS polymerization. The increased expression of HbA2 could also compensate for the reduced Hb production that is often observed in SCD. The elevated white blood cell counts in patients with SCD and BERK mice reflect inflammation that is likely to contribute to organ damage; this measurement was significantly improved in KG-M mice (Table 1). These phenotypic corrections by KLF1-GATA1 fusion proteins could be achieved in clinical use for human SCD treatment. Of note, the efficacy of HU in the treatment of SCD is generally attributed to its ability to increase HbF.59 However, it should be recognized that mean HbF ranged from 10% to 23% in patients with SCD who received HU treatment, whereas their baseline HbF was already in the range of 4% to 12%,60 inferring that there was only a moderate absolute increase in HbF of 6% to 11% upon HU treatment. In our in vitro study, we found that introduction of the medium-form KLF1-GATA1 in human SCD CD34+ cells increased mean HbA2+HbF content from 8.1% to 19.7%. However, the biological significance of this moderate elevation may lie in the cells with higher HbF and HbA2 content surviving longer in the peripheral circulation and therefore being amplified in number.61 In accordance with this, in our animal studies, we observed a greater HbA2 induction by KLF1-GATA1 in vivo than in vitro (Figure 4D; Table 1). In addition, a substantial SCD-related phenotype correction was observed in KG mice compared with mock mice.
The KLF1-GATA1 fusion proteins were designed and shown to enhance δ-globin expression through interaction with the GATA1-binding motif of the δ-globin promoter.31 We wanted to know whether the fusion proteins could have a different pattern of chromatin occupation from GATA1, especially within the human β-globin locus, which may lead to off-target effects. Chromatin immunoprecipitation sequencing showed that KLF1-GATA1 fusion protein shares a similar pattern of chromatin occupation within the human β-globin locus and at a global genomic scale with GATA1 in primary adult human erythroid progenitor cells (supplemental Figure 1, available on the Blood Web site; also see the UCSC Genome Browser [https://genome.ucsc.edu/s/mpirooz1/GATA1_chromatin immunoprecipitation]). GATA1 can function either as a transcriptional activator or repressor, depending on promoter/enhancer context and co-recruitment of other factors.62-68 For example, GATA1, BCL11A, and SOX6 co-occupy the human β-globin cluster and cooperate in suppressing γ-globin transcription in adult human erythroid progenitors65; GATA1 and NF-Y activate HbF cooperatively through their respective binding motifs in the γ-globin promoter in HUDEP2 cells.64 However, how GATA1 exerts these distinct functions remains unresolved.69
When GATA1 is fused to the KLF1 activation domain, it is possible that binding to the GATA1 sites could activate genes that are normally repressed by GATA1. Numerous genes have been reported to be regulated by GATA1 in G1E cells, with GATA2 and MYB being 2 of the GATA1-repressed genes.24,70 To investigate KLF1-GATA1’s potential activation of genes that are normally repressed by GATA1, we examined GATA2 and MYB gene expression in KLF1-GATA1–transduced erythroid cells. Medium-form KLF1-GATA1 fusion protein did not significantly affect GATA2 and MYB gene expression, whereas the long-form KLF1-GATA1 fusion protein increased GATA2 gene expression and reduced MYB gene expression (supplemental Figure 2). The differences in cell type and culture system used in our study from those used in previous reports may account for these unexpected findings. However, although KLF1-GATA1 (like GATA1) may have the ability to co-recruit other factors to regulate its target genes, because GATA1 and KLF1-GATA1 are different proteins, the factors that they co-recruit may not be identical, thereby producing different effects on target genes. This possible difference in co-recruitment represents another possible explanation of our findings that KLF1-GATA1 did not activate some GATA1-repressed genes.
GATA1 is critical for promoting the maturation of precursor cells (eg, erythroblasts and megakaryoblasts) to red blood cells and platelets71-75 and in regulating early stages of eosinophil differentiation.76 KLF1 itself is an erythroid cell–specific transcriptional factor and is essential to terminal erythroid differentiation. In this study, we found that KLF1-GATA1 fusion proteins increased δ-globin gene expression without interrupting erythroid cell differentiation, enucleation, and proliferation. In addition, KG mice demonstrated a significant improvement in hematologic parameters (compared with mock mice; Table 1) and had normal blood platelet counts (data not shown). The improvement in SCD-related phenotypes observed in KG mice was not accompanied by any notable adverse effects (eg, bodyweight loss and daily activity). These results indicate that the off-target effects of the 2 KLF1-GATA1 fusion proteins tested were minimal; however, other genes that could be regulated by KLF1-GATA1 fusion protein must be further investigated.
Although full myeloablative conditioning has been successfully used in stem-cell transplantation for pediatric patients with SCD,7 it is not suitable for adults with severe SCD who exhibit preexisting multiorgan disease.54 In the present study, we used a low dose of total body irradiation to develop sufficient myelosuppression and create enough bone marrow space to allow for a degree of hematopoiesis from donor stem cells.77,78 This donor-derived hematopoiesis has been shown sufficient to reverse the SCD phenotype.77-79 A low level of irradiation, together with rapamycin use, has been shown to suppress the immune response and prevent the occurrence of graft-versus-host disease.79,80 In our SCD mouse transplantation studies, we achieved 36% ± 6% engraftment of donor cells in the bone marrow (data not shown), which is consistent with results in a recent report.81 Because cytokine pretreatment could induce HSC differentiation and loss of long-term repopulating activity,82 in our protocol, mouse HSCs were directly incubated with lentivirus without cytokine prestimulation before gene transduction. All of these modifications, including the elimination of myeloablative conditioning and cytokine prestimulation, would make our protocol more applicable to clinical use.
We observed that the effect of medium-form KLF1-GATA1 on HbA2 induction and sickle-phenotype correction is much more profound than that of long-form KLF1-GATA1. This finding could be owing to the long-form fusion protein that consists of a full-length KLF1 and a fragment of GATA1 (amino acid residues 145-413), thereby retaining the full function of KLF1 and partial features of GATA1 and synergistically activating the β-globin promoter to activate β-globin expression.83 Thus, in KG-L mice, the beneficial effects of increasing HbA2 could be offset by a corresponding increase in HbS produced by the long-form KLF1-GATA1. The expression of Hb is controlled by the complex interaction between cis-acting sequences and trans-acting factors.84 This intricate control mechanism may explain why we observed the induction of HbF by KLF1-GATA1 fusion protein in erythroid cells cultured from human sickle HSCs but not in erythroid cells cultured from SCD mouse HSCs, in which mouse Hb was silenced and human Hb was knocked in. In addition, the increase of δ-globin expression is not reciprocal to the decrease of β-globin expression in our study. Although some studies have indicated a reciprocal relationship between γ- and β-globin gene expression,85,86 the relationship between δ- and β-globin gene expression has not been reported and requires further investigation.
In summary, this study demonstrated that 2 functional KLF1-GATA1 fusion proteins could significantly increase δ-globin expression and improve the SCD phenotype both in vivo and in vitro. Because the observed effects of the medium-form KLF1-GATA1 fusion protein were greater than those seen with the long-form fusion protein, the medium-form KLF1-GATA1 may be more suitable for SCD treatment. We reason that both KLF1-GATA1 fusion proteins would also be applicable for treatment of β-thalassemia, because the induction of both δ- and β-globin would be expected to compensate for reduced β-globin production and improve the non–α-globin/α-globin chain ratio. With their minimal off-target effects, these KLF1-GATA1 fusion proteins could prove useful as a genetic therapeutic tool for SCD and β-thalassemia.
Acknowledgments
The authors thank members of the DNA Sequencing and Genomics Core Facility (National Heart, Lung, and Blood Institute [NHLBI]) for assistance with chromatin immunoprecipitation ()-sequencing; Duck-Yeon Lee (Biochemistry Core Facility, NHLBI) for expertise and advice regarding high-performance liquid chromatography and data analysis; and the Department of Laboratory Medicine (Clinical Center, National Institutes of Health) members for their assistance with hematologic analysis.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (ZIA HL006006.).
Authorship
Contribution: J.Z. designed and performed the research, analyzed the data, and wrote the manuscript; H.L. performed the animal study and contributed to manuscript preparation; C.K. contributed to the analysis of the flow cytometry data; W.A. contributed to the quantitative-PCR studies; A.S. and P.D. performed the sickle cell imaging flow cytometry assay (SIFCA) and cell sorting; M.P. analyzed and interpreted the ChIP-sequencing results and contributed to manuscript preparation; K.C. contributed to lentiviral packaging and scientific discussion of the results; and G.P.R. conceived and participated in the design of the study, the evaluation of the results, and the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Griffin P. Rodgers, Building 10, Room 9N119, Molecular and Clinical Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Dr, Bethesda, MD 20892; e-mail: gr5n@nih.gov.
References
Author notes
The data that support the findings of this study are available on request from the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal