The most prominent morphological feature of platelets is their unusual discoid shape from which they derive their name. But why do platelets have such a unique design? In this issue of Blood, Kimmerlin et al1 show that knockout of α4A and β1 tubulin results in mice with spherical platelets that have marked defects in hemostasis and thrombosis.
In 1882, Giulio Bizzozero was evaluating flowing blood in the mesenteric vessels of rabbits and guinea pigs when he first observed a new type of tiny cell that he described as “a very thin platelet, disc-shaped, with parallel surfaces.”2 In 1965, with the advent of electron microscopy, Behnke demonstrated a peripheral microtubule bundle in human and rat platelets and proposed that this structure gave platelets the platelet discoid shape first described by Bizzozero.3 This microtubule structure was subsequently termed the marginal band. Microtubules that make up the marginal band consist of heterodimers of α- and β-tubulin subunits aligned head to tail to form protofilaments.4,5 These protofilaments laterally associate, enabling the formation of hollow rigid tubular structures. There are many isoforms of α- and β-tubulins in platelets, with α4-tubulin and β1-tubulin (whose expression is restricted to hematopoietic lineages) being among those most highly expressed.6 In the resting platelet, this marginal band is a submembranous ring of approximately 9 μm and 8 to 12 microtubules that circumscribes the platelet periphery and encircles all platelet organelles.
Despite detailed knowledge of marginal band composition and dynamics, the reason platelets are discoid has remained a mystery. One popular idea is that platelets evolved a flat shape to improve their ability to form tight aggregates by providing an extended surface for platelet-platelet interactions. This idea was bolstered by the identification of a patient with a bleeding disorder whose platelets were spherical and lacked a marginal band.7 Studies of β1-tubulin knockout mice, however, raised doubts about this premise. These mice showed reduced microtubule content and spherical platelets but nonetheless demonstrated normal bleeding times and normal adhesion under shear.8 The fact that the β1-tubulin knockout platelets retained a thin marginal band and adopted a somewhat elliptical shape kept open the question of whether platelet discoid shape promotes platelet adhesion.
The generation and extensive phenotyping of the α4A- and β1-tubulin double knockout mouse by Kimmerlin et al now address this issue. The investigators show that the double mutant has reduced platelet counts and increased platelet volumes. Erythrocytes and leukocytes appeared to be unaffected. A strength of these experiments is that the investigators evaluated single α4A-tubulin and β1-tubulin knockouts as well as wild-type and double knockout mice. Nearly all the double knockout platelets (97%) lacked discoid shape and 73% were spherical, whereas in β1-tubulin knockouts 68% lacked discoid shape and 24% were spherical. Peritoneal bleeding was observed in double knockout mice, but not in the other genotypes. Consistent with a profound bleeding abnormality, tail snip assays showed continued bleeding after 30 minutes in double knockout mice, whereas the majority of mice of the other genotypes achieved hemostasis. To rule out the possibility that enhanced bleed resulted from thrombocytopenia observed in the double knockout animals, mice were infused with romiplostim to increase their platelet counts. Enhancement of platelet counts did not reverse the bleeding phenotype. Defective hemostatic function correlated with impaired ability to form thrombi in vivo. Only a thin layer of platelets accumulated following exposure of carotid arteries to ferric chloride (FeCl3) in double knockout mice. In contrast, other mutants and wild-type mice demonstrated arterial occlusion under the same conditions. Transplantation of bone marrow from the double knockout donor mice into wild-type recipients confirmed that the defect in FeCl3-induced thrombus formation resided with spherical platelets and not in the vascular wall. The double knockout mice were also protected from thrombotic death caused by collagen and adrenaline infusion.
Why are spherical platelets dysfunctional? Surface concentration of major platelet surface glycoprotein adhesion receptors was unchanged. Rigorous evaluation of platelet activation showed that platelets continued to activate normally and to secrete α-granules and dense granules in response to standard platelet agonists such as collagen and thrombin. Platelet aggregation was not decreased and, in fact, was slightly increased compared with that in wild-type platelets. Platelet spreading under static conditions was also increased in spherical platelets. Nonetheless, when tested under flow conditions, adhesion to immobilized von Willebrand factor was impaired in double knockout platelets, which detached more easily than the other genotypes from immobilized von Willebrand factor. These abnormalities in adhesion were observed under conditions mimicking both arterial and venous shear rates. Delayed thrombus formation of double knockout platelets was also observed when they were flowed over collagen. This defect in platelet adhesion to collagen persisted despite correction of thrombocytopenia via administration of romiplostim. These studies indicated that the bleeding and thrombus formation defect in double knockout mice did not result from an activation defect, but rather from a defect in adherence to the vascular wall under flow conditions.
Platelets have evolved several unique features to enhance their performance as agents of hemostasis and thrombosis. They are anucleate, decreasing their density such that they partition close to the vessel wall while circulating in blood. They expose a high density of adhesive glycoproteins on their surface. They are replete with granules filled with procoagulant cargos. And now, with the work of Kimmerlin et al, we can add their discoid shape as a feature of their prothrombotic capacity. By increasing the area of adhesive membrane available for attachment to an injured surface, discoid shape appears to promote the formation of durable platelet-rich thrombi capable of resisting forces of shear and push experienced by a stationary clot subjected to high flow rates in the circulation (see figure). A more detailed account of platelet adhesion of discoid vs spherical platelets will be required to understand the extent to which the flat surface of the discoid platelet promotes thrombosis and whether nonhemostatic platelet functions also benefit from discoid shape. Yet these studies provide the strongest support yet that its discoid shape enhances platelet interactions with the vessel wall and neighboring platelets to strengthen developing clots.
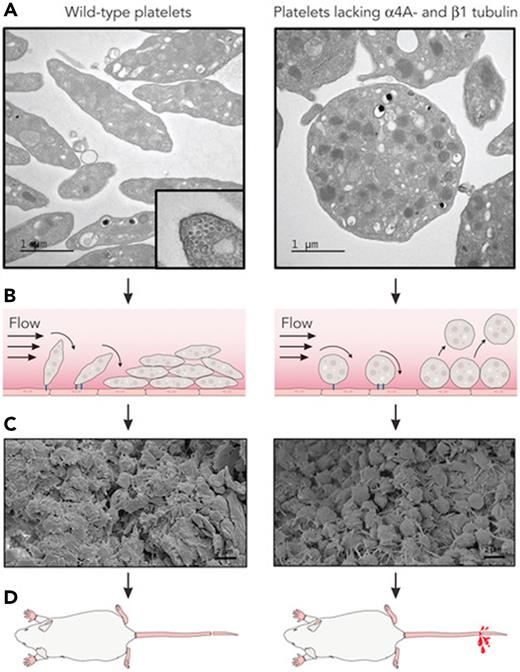
Discoid shape promotes durable thrombi. (A) Platelets that lack α4A-and β1-tubulin demonstrate a spherical morphology and lack the microtubule-derived marginal band, a cross-section of which is shown in the left panel. (B) Under flow conditions, the discoid shape of wild-type platelets provides an increased surface area for attachment to the vessel wall (black line) and to each other. By comparison, spherical platelets form looser attachments that are prone to give way to flow. (C) As a result, thrombi formed by discoid platelets are more compact. (D) The friability of thrombi formed by spherical platelets results in increased bleeding. (Electron micrographs were extracted from their original Figures 1 and 3 in Kimmerlin et al that begins on page 2290. Professional illustration by Patrick Lane, ScEYEnce Studios.)
Discoid shape promotes durable thrombi. (A) Platelets that lack α4A-and β1-tubulin demonstrate a spherical morphology and lack the microtubule-derived marginal band, a cross-section of which is shown in the left panel. (B) Under flow conditions, the discoid shape of wild-type platelets provides an increased surface area for attachment to the vessel wall (black line) and to each other. By comparison, spherical platelets form looser attachments that are prone to give way to flow. (C) As a result, thrombi formed by discoid platelets are more compact. (D) The friability of thrombi formed by spherical platelets results in increased bleeding. (Electron micrographs were extracted from their original Figures 1 and 3 in Kimmerlin et al that begins on page 2290. Professional illustration by Patrick Lane, ScEYEnce Studios.)
Conflict-of-interest disclosure: R.F. is a founder and consultant for Platelet Diagnostics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal