In this issue of Blood, Wilkens et al describe a new system for manufacturing ex vivo Notch1-activated CD4+ chimeric antigen receptor (CAR) T cells with enhanced cytokine production, expansion, helper T-cell function, and anticancer activity.1
CAR-expressing T cells have emerged as essential treatment options for patients with relapsed or refractory B-cell malignancies, and indications for their use in other diseases are being actively explored. The success and broader applicability of CAR T-cell therapies are limited by several factors, including insufficient T-cell expansion and acquired T-cell dysfunction.2 The study by Wilkens et al uncovered a new role for ex vivo Notch activation to enhance antitumor CAR T-cell activity.
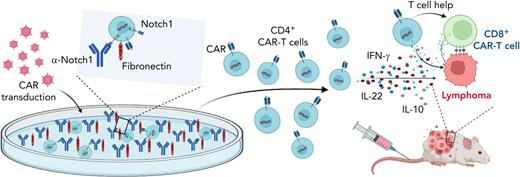
Ex vivo ligand-mediated activation of Notch signaling was first developed to increase hematopoietic stem and progenitor cell expansion and to sustain the development of Notch-dependent immature T cells.3,4 Multiple effects of Notch signaling have been described in mature post-thymic T cells. There is a growing consensus around the following overarching functions of Notch in support of immune activation: enhanced inflammatory cytokine production, increased cytotoxic effector potential, and decreased activity of regulatory T cells.5 Moreover, Kondo and colleagues6,7 first reported that exposure to Notch ligands could induce the formation of activated T cells or CAR T cells with stem-cell memory characteristics and with improved in vivo persistence and antitumor activity. Wilkens et al now demonstrate that induction of Notch1-mediated signals during the manufacture of human CD4+ CAR T cells maintained a less differentiated T-cell phenotype. In addition, Notch signaling promoted proinflammatory cytokine production at least in part via increased expression of the transcription factors AhR and c-Maf, although additional mechanisms of action may still remain to be discovered. Interestingly, distinct populations of Notch1-activated CD4+ CAR T cells were responsible for the production of interferon-γ (IFN-γ) and interleukin-22 (IL-22). When transferred into xenograft tumor models, Notch1-activated CD4+ CAR T cells proliferated more robustly in vivo and induced more efficient tumor regression than control CAR T cells, although these effects were transient when Notch1-activated CD4+ CAR T cells were used in isolation. When co-transferred with CD8+CD19-specific CAR T cells, however, Notch-activated CD4+ CAR T cells provided increased helper function, which led to more efficient durable regression of B-cell lymphoma xenografts (see figure).
Notch signaling promotes CD4+ CAR T-cell persistence and helper function. Figure shows that culturing of CD4+ T cells on fibronectin and anti-Notch1 antibody-coated plates during CAR T-cell transduction and expansion activated Notch signaling. Notch1-activated CD4+ CAR T cells produced more proinflammatory cytokines and had enhanced in vivo persistence as well as helper T-cell function in lymphoma xenograft models. Created with BioRender.com.
Notch signaling promotes CD4+ CAR T-cell persistence and helper function. Figure shows that culturing of CD4+ T cells on fibronectin and anti-Notch1 antibody-coated plates during CAR T-cell transduction and expansion activated Notch signaling. Notch1-activated CD4+ CAR T cells produced more proinflammatory cytokines and had enhanced in vivo persistence as well as helper T-cell function in lymphoma xenograft models. Created with BioRender.com.
In addition to describing the therapeutic potential of Notch1-activated CD4+ CAR T cells, Wilkens et al developed an antibody-based strategy to agonize individual Notch receptors, a useful system that could provide insights into other Notch-mediated processes. Notch receptors are transmembrane proteins that normally get activated after binding to fixed ligands of the Jagged or Delta-like family on adjacent cells. Ligand-receptor interactions induce a pulling force on the Notch receptor that leads to conformational changes, enabling access to the ADAM10 metalloprotease followed by proteolytic activation, release of the Notch intracellular domain, and downstream transcriptional effects.8 Most existing ex vivo strategies for Notch agonism rely on coculture with cell-bound or plate-bound Notch ligands that can activate all 4 Notch receptors (Notch1-Notch4). By using plates coated with anti-Notch1 antibodies and fibronectin, the authors developed a system that generates enough shear force to activate the Notch1 receptor with potency that is similar to that of previous ligand-based assays. In the future, antibodies specific for other Notch receptors could be screened for their capacity to induce ex vivo Notch activation, which would provide the opportunity to systematically probe the impact of activating individual Notch receptors in target cell types. Interestingly, it may also be possible to develop agonistic antibodies binding specific Notch receptor domains that induce conformational changes and activate Notch signaling without additional mechanical forces—a potential path toward the development of in vivo Notch agonists.9 For now, antibody-based approaches should provide a cost-effective cell-free scalable method of ex vivo Notch agonism ideally suited for the manufacture of clinical-grade cellular products.
The study by Wilkens et al establishes a potentially valuable role for Notch agonism in CD4+ CAR T-cell expansion and differentiation. Although most clinically approved CAR T-cell products are generated from unselected T-cell populations collected during leukapheresis, there are precedents for separate manufacturing of CD4+ and CD8+ CAR T cells, indicating that this type of approach would have real-world feasibility.10 The effects of Notch agonism on CD4+ CAR T cells illustrate the potential impact of this strategy, because different T-cell subsets could be transduced and expanded under distinct conditions. Further studies are needed to evaluate the effects of Notch agonism during the manufacture of CD8+ CAR T cells, which might have additive or synergistic effects on antitumor immunity when combined with Notch-agonized CD4+ CAR T cells. Initial observations about the capacity of Notch activation to induce differentiation into stem-cell memory T cells included CD8+ T cells; conversely, Notch signaling was also reported to promote differentiation of CD8+ T cells into short-lived effector cells during anti-infectious responses.5-7 Thus, the jury is still out about all the effects of Notch in CD8+ conventional T cells and CD8+ CAR T cells. To what extent Notch has a direct or indirect impact on CD8+ T cells via CD4+ helper T-cell functions also needs to be considered. Additional work will need to evaluate the long-term consequences of a transient pulse of Notch signaling in CAR T cells and the ideal timing for Notch agonism ex vivo and in vivo (ie, during manufacturing and/or after administration to recipients of CAR T cells). The increased cytokine production and proliferation observed with Notch1-agonized CD4+ CAR T cells raise concerns for increased risk of toxicities, including cytokine release syndrome. In future clinical translations, it will be critical to carefully examine patients for any adverse effects of these interventions although the transient nature of Notch agonism may be important to mitigate these risks and to avoid consequences of chronic Notch activation in T cells.
Altogether, this work by Wilkens et al provides valuable new insights into the role of Notch signaling in cancer immunology and further supports a proinflammatory role for Notch activation in CD4+ T cells. Most importantly, it demonstrates that Notch receptor-specific agonism can be used to increase the anticancer efficacy of CAR T cells. Such innovative strategies could facilitate the development of more potent CAR T cells and improve the clinical outcomes for patients with cancer.
Conflict-of-interest disclosure: I.M. has received research funding (unrelated to the contents of this commentary) from Regeneron and Genentech and is a member of the scientific advisory board for Garuda Therapeutics. The remaining author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal