In this issue of Blood, Fujisawa et al discuss their discovery that tet methylcytosine dioxygenase 2 (TET2) mutations within the B cells of the tumor microenvironment are necessary for facilitating TET2-mutated T follicular helper (TFH) lymphoma.1 Through an elegant series of experiments, the investigators determined the mechanism of disease pathogenesis and introduced potential therapeutic strategies to target this biology.
Morphologically, it has been long recognized that angioimmunoblastic T-cell lymphoma (AITL), derived from germinal center TFH cells, has a distinctive and complex tumor microenvironment. Just as its name suggests, this disease is characterized by arborization of vessels as well as an influx of polymorphic immune cells. Frequently, a small clone of B lymphocytes or plasma cells is seen concurrently alongside the clonal T-cell population. How these abnormal cells interact with one another within the germinal center and their importance in the development of T-cell lymphoma is less well understood.
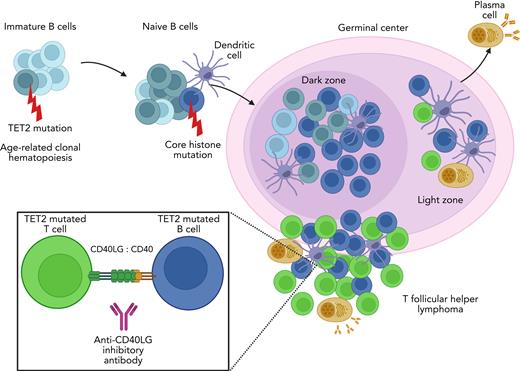
In normal physiologic conditions, the germinal center of the lymph node is a dynamic microanatomic compartment where naïve B lymphocytes undergo affinity maturation, proliferation, and differentiation to produce a diverse set of antibodies.2 The germinal center itself is polarized into subcompartments: the light zone and dark zone. B cells enter the dark zone where they undergo somatic hypermutation and proliferation. As B cells migrate through the light zone, they encounter antigen-presenting follicular dendritic cells and TFH cells, the latter of which engage via the CD40-CD40LG interaction. This touch point plays an important role in regulating both the adaptive and cell-mediated immune responses through activation of mTOR, IL2, STAT5, and IFN-γ signaling in T cells.3 From here, B cells may repeatedly recycle back to the light and dark zones or exit the germinal center as memory B cells or plasma cells. A number of epigenetic and transcriptional controls, including histone methylation by EZH2, BCL6, and IRF4, are crucial for enabling these processes to occur.
TET2 catalyzes the conversion of methylcytosine to 5-hydroxymethylcytosine on DNA, which is a required initial step in DNA demethylation. TET2 functions as a tumor suppressor; however, inactivating mutations in this gene lead to hypermethylation of DNA at promoter regions, coding and noncoding sequences, and 5′ untranslated regions. TET2 mutations have long been identified in a number of malignancies and, along with RHOA mutations, constitute a hallmark finding in AITL.4,5 Interestingly, TET2 mutations have also been observed in germinal center–derived B-cell lymphomas.6
In the article by Fujisawa et al, the investigators used innovative technologies to shift the focus from the malignant T cells to the B cells within the microenvironment. In doing so, they discovered that TET2 mutations within B cells in the tumor microenvironment are required for the generation and expansion of malignant TFH cells. Strikingly, the investigators found that when G17V-RHOA mice were crossed with TET2-mutated mice, the addition of TET2-mutated immune cells to the microenvironment was necessary for the formation of TFH lymphoma. In contrast, when G17V-RHOA–mutant mice were crossed with only T cells harboring TET2 mutations, only a small fraction of the mice developed lymphoma, and if they did, it was after a much longer interval of time. The authors propose that age-related clonal hematopoiesis may be responsible for the generation of somatic TET2 mutations in both B and T cells (see figure). To better understand events that follow, the investigators used transgenic mouse models, single-cell RNA sequencing, and interactome studies to deconvolute the complex interaction within the germinal center and dissect out differences between T, B, plasma, and myeloid cells. They noted that TET2 mutations in B cells seemed to be an early event that would lead to clonal evolution of B cells in this setting. Using trajectory analysis, they found that these abnormal B cells have features intermediate between the light zone and dark zone with evidence of both activation and proliferation. Using 3D structural analysis, they discovered that additional mutations acquired in these tet2-deficient B cells, but not TFH cells, include derangements in other epigenetic marks, specifically in core histones (HIST1H1C, HIST1H3A, HIT2H3D), further contributing to the expansion of these abnormal B cells. These mutations were identified in the mouse models and validated in primary human AITL samples. The authors revealed that the interaction of TET2-mutated B cells with abnormally activated malignant T cells aided in the evolution of a specific gene expression profile in these tet2-deficient B cells, which generated a unique signature from what is seen in other TET2-mutated B-cell lymphomas.
TET2-mutated B- and T-cell niches within the germinal center promote TFH lymphomagenesis. Immature B cells acquire TET2 mutations through age-related clonal hematopoiesis. This leads to clonal expansion of B cells that later acquire additional mutations such as those in core histones. These TET2-mutated B cells proliferate within the dark zone of the germinal center. As they traverse to the light zone, B cells and T cells interact with follicular dendritic cells for antigen capture and presentation. Increased CD40 expression on B cells promotes interaction and stimulation of TFH cells via the CD40LG. TFH lymphoma develops in niches with B cells that have features intermediate between the light and dark zones. Breaking the CD40-CD40LG interaction with anti-CD40LG inhibitory antibody reduces the growth of TFH lymphoma and prolongs survival in mouse models of this disease.
TET2-mutated B- and T-cell niches within the germinal center promote TFH lymphomagenesis. Immature B cells acquire TET2 mutations through age-related clonal hematopoiesis. This leads to clonal expansion of B cells that later acquire additional mutations such as those in core histones. These TET2-mutated B cells proliferate within the dark zone of the germinal center. As they traverse to the light zone, B cells and T cells interact with follicular dendritic cells for antigen capture and presentation. Increased CD40 expression on B cells promotes interaction and stimulation of TFH cells via the CD40LG. TFH lymphoma develops in niches with B cells that have features intermediate between the light and dark zones. Breaking the CD40-CD40LG interaction with anti-CD40LG inhibitory antibody reduces the growth of TFH lymphoma and prolongs survival in mouse models of this disease.
The crosstalk between B and T cells further accelerates the development of TFH lymphoma. Analysis of intercellular ligand-receptor interactions between germinal center B cells and TFH tumor cells revealed specific interactions that were enhanced between these cell types, including Cd40-Cd40lg. The increase in Cd40 expression was observed in these abnormal B cells before lymphoma developed, suggesting that the interaction between Cd40-Cd40lg and resulting downstream signaling enhances the development and expansion of T-cell lymphoma. Leveraging this finding, the investigators treated an AITL mouse model with anti-Cd40lg inhibitory antibody, which prolonged survival in the treated mice.
AITL is the second most common peripheral T-cell lymphoma and has a dismal 5-year overall survival of only 31.6%.7 It is usually CD30 negative, which correlates with reduced activity of brentuximab vedotin in this disease. Efforts to improve outcomes have focused on targeting epigenetic vulnerabilities8,9 or the mTOR/PI3K pathway,10 including the National Clinical Trials Network frontline study (NCT04803201). The addition of a monoclonal antibody that targets the biological drivers of this disease has the potential to enhance ongoing efforts to improve outcomes.
Fujisawa et al used sophisticated approaches to tease out the intricate dance between AITL and its heterogeneous microenvironment. They determined that germinal center B cells undergo independent clonal expansion and selection that ultimately leads to increased CD40 signaling. This crucial event enhances niche formation between germinal center B and T cells driving TET2/RHOA-mutated T cells toward malignant transformation. What is still unknown is the contribution of plasma cells in this niche, what specific role mutations in core histones are playing, and how Epstein-Barr virus infection may be driving some of these events. The findings presented in the article by Fujisawa et al culminated in identifying that breaking the CD40-CD40LG interaction could have therapeutic benefit by opening a window of opportunity for advancing treatment options in AITL.
Conflict-of-interest disclosure: J.E.A. has received research funding from Appia Pharmaceuticals and honoraria from Daiichi Sankyo and AstraZeneca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal