TO THE EDITOR:
Patients with β-thalassemia show 5-fold increase in age-standardized lethality due to SARS-CoV-2 infection, representing a high-risk population compared with age- and sex-matched healthy subjects.1 Vaccination against SARS-CoV-2 is crucial to reduce mortality and morbidity of frail patients.2 Up to now, limited data have been available on the responses of patients with β-thalassemia immunized with anti–SARS-CoV-2 mRNA vaccines.3
The main aim of the present prospective multicenter study was to evaluate the immunogenicity of the SARS-CoV-2 mRNA vaccine BNT162b2 in patients with transfusion-dependent β-thalassemia (TDT). Patients with TDT (n = 154), vaccinated within the Nationwide Vaccination Program in Italy, were enrolled in 7 comprehensive Italian centers for hemoglobinopathies. Serum samples were collected before vaccination (T0), 2 weeks after the second dose (T1), 12 weeks after the second dose (T2), before the third dose (T3), and at 4 and 12 weeks after the third dose (respectively, T4 and T5) (supplemental Figure 1A, available on the Blood website). A group of healthcare workers (HCWs) enrolled at INMI L. Spallanzani served as control group. Previous and breakthrough SARS-CoV-2 infections were diagnosed by a positive molecular reverse transcriptase polymerase chain reaction test or seroconversion to antinucleocapsid antibodies. The response to vaccination was evaluated by quantifying anti–spike protein receptor-binding domain (RBD) antibodies (Abbott Laboratories, Wiesbaden, Germany). The spike protein–specific T-cell response was evaluated in a subset of patients (n = 12) by an interferon-γ–releasing assay performed on whole blood. In the same group of patients, spike protein–specific memory B cells were detected and quantified by flow cytometry or ELISpot. The study was approved by the Comitato Etico dell’Istituto Nazionale per le Malattie, Infettive Lazzaro Spallanzani IRCCS as the National Review Committee Board for the COVID-19 pandemic in Italy, and registered with clinicaltrials.gov (NCT05157256). Overall, 154 patients with TDT and 82 HCWs were enrolled in this study; all subjects received the mRNA BNT162b2 vaccine. Demographic and clinical patients’ characteristics are summarized in Table 1. After vaccinations, no serious adverse events related to vaccination were reported in the study population. Moreover, no COVID-related hospitalization or death was recorded in the study population.
Main characteristics of subjects included in the analysis
| Subjects' characteristics . | Value∗ . |
|---|---|
| HCWs | 82 |
| Men | 21 (25.6%) |
| Women | 61 (74.4%) |
| Age, y | 45.0 (35.0-53.0) |
| SARS-CoV-2 | |
| Naïve | 82 (100%) |
| Previously infected | — |
| TDT patients | 154 |
| Men | 61 (39.6%) |
| Women | 93 (60.4%) |
| Age, y | 44.0 (37.0-51.0) |
| Splenectomized | 83 (53.9%) |
| ICT | 154 |
| DFX | 80 (51.9%) |
| DFP | 20 (13.0%) |
| DFO | 18 (11.7%) |
| Combined | 31 (20.1%) |
| Not available | 5 (3.2%) |
| HU | 17 (11.0%) |
| SARS-CoV-2 | |
| Naïve | 132 (85.7%) |
| Previously infected | 22 (14.3%) |
| Subjects' characteristics . | Value∗ . |
|---|---|
| HCWs | 82 |
| Men | 21 (25.6%) |
| Women | 61 (74.4%) |
| Age, y | 45.0 (35.0-53.0) |
| SARS-CoV-2 | |
| Naïve | 82 (100%) |
| Previously infected | — |
| TDT patients | 154 |
| Men | 61 (39.6%) |
| Women | 93 (60.4%) |
| Age, y | 44.0 (37.0-51.0) |
| Splenectomized | 83 (53.9%) |
| ICT | 154 |
| DFX | 80 (51.9%) |
| DFP | 20 (13.0%) |
| DFO | 18 (11.7%) |
| Combined | 31 (20.1%) |
| Not available | 5 (3.2%) |
| HU | 17 (11.0%) |
| SARS-CoV-2 | |
| Naïve | 132 (85.7%) |
| Previously infected | 22 (14.3%) |
DFO, deferoxamine; DFP, deferiprone; DFX, deferasirox; HCWs, healthcare workers; HU, hydroxyurea; ICT, iron chelation therapy; TDT, transfusion-dependent β-thalassemia.
Values are n, n (%), or median (interquartile range).
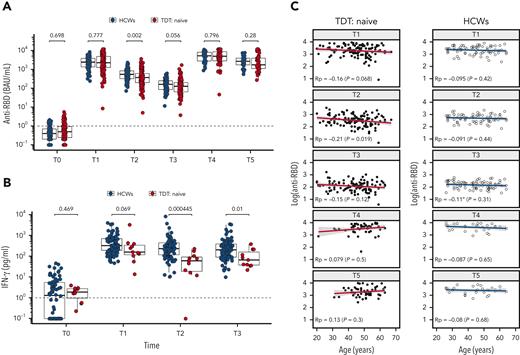
As shown in Figure 1A, after 2 doses (T1), infection-naïve patients TDT (n = 132) showed an optimal rate of seroconversion to anti-RBD IgG (131/132, 99.2%), similarly to the control HCWs (82/82, 100%). The anti-RBD titers of infection-naïve TDT and HCW subjects were similar at T1, thus confirming excellent response to the vaccine. At T2, however, 12 weeks after the second dose, anti-RBD antibodies were significantly lower in patients with TDT than in controls (estimated difference, 148 binding arbritary units (BAU)/mL; confidence interval, 55-244 BAU/mL). At T3, just before the third dose, anti-RBD antibodies were lower in patients with TDT than in controls without reaching statistically significant difference (P = .056; estimated difference, 26.6 BAU/mL; confidence interval, −0.7 to 55 BAU/mL). Thus, notwithstanding the optimal response to the vaccine, antibody titers declined more rapidly in patients with TDT than in HCWs after the first 2 doses. Similar kinetics was observed for the spike protein–specific T- and B-cell responses (Figure 1B; supplemental Figure 1B), confirming a good immune response early after vaccination that decreases faster over time in patients with TDT than in age-matched HCWs. The third dose was able to improve the immune response of patients with TDT (T4 vs T3, P < .0001), reaching 100% seropositivity rate, with a similar anti-RBD titer in the TDT and HCW groups (T4) (Figure 1B). Of note, the level of anti-RBD antibodies was higher at T4, 4 weeks after the third dose, than at T1 (P < .0001), demonstrating the beneficial effect of the third dose in achieving an optimal antibody titer in patients with TDT. Most importantly, at T5, 12 weeks after the third dose, antibody titers were higher than at T2 in both patients with TDT and HCWs, suggesting that the third vaccine dose may be able to generate an antibody production that is sustained for a longer time. We also evaluated the immune response of a small population of patients with TDT (n = 22) who received the BNT162b2 vaccine 1 to 3 months after SARS-CoV-2 infection. As shown in supplemental Figure 2A, previously infected patients with TDT showed significantly higher anti-RBD titer levels in response to vaccination at T1, T2, and T3 compared with infection-naïve patients with TDT. There was no difference at T4 and T5, corresponding respectively to 4 and 12 weeks after the booster dose (supplemental Figure 2A). Thus, we confirm the strength of hybrid immunity4 in patients with TDT, but we also show that, after the booster dose, equally high antibody levels are produced in patients never infected by SARS-CoV-2.
Kinetics of anti-RBD Abs and spike-specific T-cell response in patients with TDT compared with control subjects. (A) Comparison between health care workers (HCWs; open circles) and patients with transfusion-dependent β-thalassemia (TDT; red circles) naïve to SARS-CoV-2 at each sampling time. (B) Spike-specific T cells (measured by interferon (IFN) γ release after specific stimulation) in the peripheral blood of control HCWs (open circles) and patients with TDT (red circles) measured at the indicated time points. (C) Correlation between anti–spike RBD antibodies and age in patients with TDT and healthy subjects (HCWs) naïve to SARS-CoV-2. T0, before vaccination; T1, 2 weeks after the second dose; T2, 12 weeks after the second dose; T3, before the third dose; T4, 4 weeks after the third dose; T5, 12 weeks after the third dose; Rp, Pearson’s correlation coefficient.
Kinetics of anti-RBD Abs and spike-specific T-cell response in patients with TDT compared with control subjects. (A) Comparison between health care workers (HCWs; open circles) and patients with transfusion-dependent β-thalassemia (TDT; red circles) naïve to SARS-CoV-2 at each sampling time. (B) Spike-specific T cells (measured by interferon (IFN) γ release after specific stimulation) in the peripheral blood of control HCWs (open circles) and patients with TDT (red circles) measured at the indicated time points. (C) Correlation between anti–spike RBD antibodies and age in patients with TDT and healthy subjects (HCWs) naïve to SARS-CoV-2. T0, before vaccination; T1, 2 weeks after the second dose; T2, 12 weeks after the second dose; T3, before the third dose; T4, 4 weeks after the third dose; T5, 12 weeks after the third dose; Rp, Pearson’s correlation coefficient.
We then assessed the possible impact of clinical variables on the humoral response. We first compared anti-RBD antibodies by age, sex, splenectomy, and chelation therapy. We found no evidence for any association at each time point except for age (Figure 1C; supplemental Figure 2). As shown in Figure 1C, we observed a slight negative correlation (Pearson correlation coefficient, Rp) between age and anti-RBD titer at T1 (T1: Rp = −0.16; P = .068), reaching statistical significance at T2, corresponding to 12 weeks after the second vaccine dose (T2: Rp = −0.21; P = .019). This correlation was lost at later time points and after the third vaccination. The behavior of anti-RBD antibody response to BNT162b2 vaccination in patients with TDT was similar to that reported in healthy subjects over 80 years of age,5-8 for whom the effect of age disappears after the third dose and further ones.5 Of note, evidence of premature aging of the immune system has been reported in patients with TDT.9-11 This might be related to different factors, including multiple transfusions as alloantigen stimulation associated or iron overload, negatively affecting TH response. Indeed, TDT patients have been shown to have (1) reduced CD4/CD8 ratio, (2) increased circulating interleukin (IL)-17 and transforming growth factor β with possible detrimental effect on T-cell immune response, and (3) reduction in IL-2 and interferon-γ production by activated lymphocytes from patients with TDT compared with healthy control subjects.12-14 A similar immunosenescent profile has also been invoked for the inverse relationship between neutralizing response and age in healthy elderly subjects, who are characterized by restriction of T- and B-cell repertoires15,16 and by general defects in CD4 and CD8 T-cell activation, differentiation, and function (proliferation and cytokines production), hindering a protective long-lasting immune response.17 Further analysis focusing on the expression of inhibitory/senescent markers on T cells could help in clarifying their role in dampening the strength as well as the persistence of vaccine-induced immunity both in naïve and in previously SARS-CoV-2–infected patients with TDT.
In conclusion, our data seem to indicate that immune response of patients with TDT to SARS-CoV-2 vaccination is similar to that reported in healthy elderly subjects, suggesting a premature aging of the immune system of patients with TDT. Our results demonstrate the relevance of a third dose of mRNA vaccine for patients with TDT, who represent a population at high risk of developing severe complications and fatal events related to SARS-CoV-2 infection. Antibodies, produced immediately after vaccination, are the product of short-lived plasmablasts, whereas maintenance of antibody concentrations over time depends on the function and number of long-lived plasma cells.18 The third vaccine dose is therefore needed to increase the number of long-lived plasma cells in patients with TDT, thus ensuring more effective protection, as shown for the elderly. It remains to be fully elucidated whether the third vaccine dose also improves the maintenance of memory T- and B-cell responses over time.
Acknowledgments
The authors thank the ForAnemia Foundation (Genova, Italy) for methodologic support, the Associazione per la Lotta alla Talassemia R. Vullo (Ferrara, Italy), and Giulia Albasini for logistical support.
The study was supported by the Italian Ministry of Health (Ricerca Corrente to INMI, Linea 1).
Authorship
Contribution: G.L.F. and F.L. designed the research; R.G., M. Fortini, S.B., R.D., S.P., M. Casale, A.M., L.P., E.T., M. Caminati, F.M., and L.D.F. managed the patients; C.A., R.C., E.T., E.P.M., F.C., V.P., M. Francalancia, and V.M. performed research; R.C., C.A., and B.G. analyzed and interpreted data; B.G. performed statistical analysis; C.A., R.C., V.M.P., G.L.F., F.L., and L.D.F. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.The current affiliation for C.A. is Department of Pediatric Hematology and Oncology, IRCCS Bambino Gesù Children's Hospital, Rome, Italy.
Correspondence: Gian Luca Forni, Centro della Microcitemia e delle Anemie, Congenite, Ospedale Galliera, Via Volta 6, Genova 16128, Italy; e-mail: gianluca.forni@galliera.it.
References
Author notes
∗R.C. and C.A. contributed equally to this work as first authors.
Data will be available on demand.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal