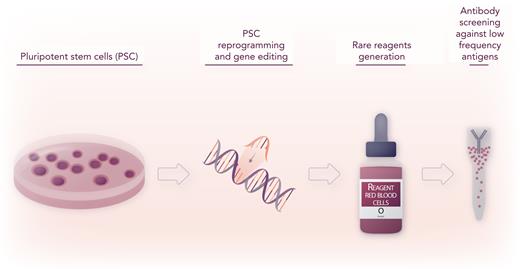
In this issue of Blood, An et al1 describe a new method to generate rare red blood cell (RBC) reagents for antibody screening in the pre-transfusion laboratory work up to identify antibodies against low prevalence variants of RH antigens. Using a combination of human induced pluripotent stem cell (iPSC) reprogramming and gene editing, the authors have engineered RBCs lacking the RH locus and expressing low-prevalence antigenic variants. Rh-engineered RBCs are compatible with standard laboratory assays and can detect the presence of plasma alloantibodies against low-prevalence allelic variants.
Chronic RBC transfusion is a cornerstone in the treatment of patients with hemoglobinopathies or deficient erythropoiesis. Development of alloantibodies against polymorphisms in more than 350 RBC antigens remains a major safety concern.2 These alloantibodies to RBC antigens frequently result in acute or delayed hemolytic transfusion reactions that can be life threatening. The development of alloantibodies is especially a major concern in patients with sickle cell disease (SCD) owing to the large number of transfusions they receive and their genetic diversity for blood groups.3
Out of all antigenic variants, diversity in the expression of the RH locus proteins has demonstrated to be a major source of immunogenicity, alloantibody formation, and post-transfusion hemolysis.4 Immunogenic RH variants result from a large number of single-nucleotide polymorphisms and gene rearrangements.5 The clinical relevance of these immunogenic variants has been demonstrated. Protocols of extended Rh typing and matching for the RH D, C, c, E, and e antigens and the K antigen in the Kell system have decreased, but not eliminated, the frequency of post-transfusion alloimmunization against Rh antigenic variants.6 Some RH genetic variants result from the loss of high-prevalence Rh antigens and others result from the gain of antigenic sequences, found more frequently in patients of Sub-Saharan ancestry.7 Many of these genetic variants associate with alloimmunization and immune-mediated hemolysis.
Prevention of alloimmunization and/or antibody-mediated hemolysis is based on a combined approach of phenotypic and genotypic matching and frequent pre-transfusion testing of preformed antibodies against clinically significant RBC antigens. Alloantibody detection includes incubation of the patient’s plasma with extensively phenotyped RBCs preceding an antiglobulin test. If screening RBCs identify the presence of antibodies, additional testing with customized panels of reagent RBCs is to be performed to characterize their specificity.
Alloantibody screening and identification require testing of patient plasma within 3 days of the scheduled transfusion if the patient has been transfused or has been pregnant in the preceding 3 months, or there is a history of previously identified antibodies.8 Successful antibody identification requires 3 single-donor antigen-negative cells that do not react to rule out and 3 single-donor antigen-positive cells to rule in each of the antigens against which antibodies are identified. Furthermore, the need of RBCs expressing or not the antigen in question is frequently not met by existing commercial panels, limiting the detection of these antibodies to sophisticated immunohematology reference laboratories.
In this study, An et al address these challenges by generating human iPSCs by reprogramming rare donor cells, nuclease-mediated gene editing, and cDNA insertion of specific Rh allelic sequences in the safe-harbor AAVS1 locus, followed by directed differentiation of iPSC to the erythropoietic lineage9 and ficin treatment to enhance the expression of Rh antigens. As a result, the investigators convincingly produced a renewable source of RBCs derived from iPSCs with unique Rh phenotypes, referred to as iRBCs,1 compatible with worldwide-used gel column agglutination laboratory assays for anti-Rh group antibody identification (see figure). As a first token of the power of their approach, the investigators generated otherwise inaccessible stomatocytic Rh-null RBCs and RhCE-deficient D RBCs, useful to screen antibodies against Rh antigens, as well as other customized iRBCs expressing rare Rh antigens,5 and validated them with real-life patient plasma tests. The investigators convincingly present data showing that ficin-treated iRBCs are similar to donor-derived RBCs in their ability to detect anti–Rh antigen antibodies and do not agglutinate or cause false positive reactions when incubated with plasma containing no antibodies or antibodies directed against non-Rh antigens. Use of iRBCs with rare or uncommon Rh phenotypes such as e+hr+hrS− and e+hrB−hrS+ could aid the identification of anti–Rh antibody specificity. In cases of a negative antibody test but a positive crossmatch, customized iRBCs were proven to be valuable to demonstrate the presence of antibodies against low-prevalence antigens such as V/VS, Go(a), and DAK.1
Pluripotent stem cell reprogramming and gene editing can generate rare reagents for precision identification of antibodies against low-prevalence antigen variants. Professional illustration by Somersault18:24.
Pluripotent stem cell reprogramming and gene editing can generate rare reagents for precision identification of antibodies against low-prevalence antigen variants. Professional illustration by Somersault18:24.
However, unlike donor-derived RBCs, the cytokine-driven erythroid differentiation program generates RBCs derived from primitive erythropoiesis programs. Therefore, they do not express many of the antigens found in donor-derived RBCs (ie, MNS, Kidd, Duffy, and Lutheran families), making them unsuitable to rule in antibodies to non-Rh antigens. They are in their vast majority nucleated and express high levels of embryonic and fetal globin chains and therefore show stronger oxygen-binding affinity. Interestingly, Rh-null iRBCs have a lower elongation index than adult progenitor-derived RBCs but not lower than wild-type iRBCs, suggesting that the hemolysis found in patients with Rh-null genotypes5 may also depend on in vivo factors not recapitulated by ektacytometry. Finally, the overall lifespan of iRBCs stored in conventional preservation buffers is reduced. The investigators point out to the need of development of optimized preservative approaches that may allow longer storage of iRBCs for commercialization and applicability in the pre-transfusion testing for patients with SCD and other chronically transfused patients worldwide. These issues can be significantly prevented by the use of nascent, yet inefficient, methods of definitive erythropoiesis differentiation of iPSCs.10
Donor-derived blood transfusion remains a cornerstone in the treatment of hematologic patients. For more than 120 years, transfusion medicine has advanced through the generation of ingenious, clinically relevant reagent tools to make transfusion safer, efficient, and practical. Advances in medicine have made precision diagnostics and therapeutics a reality. The transfusion laboratory is not falling behind. The era of genomics is generating new algorithms of blood matching and many chronically transfused patients benefit from personalized transfusion approaches. Precision alloantibody identification complements phenotype and genotype matching to advance transfusion safety.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal