In this issue of Blood, Zhu et al1 have identified vasoactive intestinal peptide (VIP) expression on plasmacytoid dendritic cells (pDCs) as an important mechanism that regulates the alloreactivity of allogeneic T cells and mitigates the severity of acute graft-versus-host disease (aGVHD).
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative therapy for a wide variety of hematologic malignancies as well as some nonmalignant blood disorders. However, about 20% of patients undergoing allo-HCT develop aGVHD, which remains the leading cause of treatment-related morbidity and mortality. Both host and donor immune modulatory mechanisms have been identified that can lessen the severity of GVHD. pDCs are a rare population of cells that mounts potent immune responses against viral infections and are a major source of type I interferons (IFNs). Previously, pDCs were shown to attenuate allogeneic T-cell activation by inducing interleukin 10 (IL-10) expression in regulatory T cells (Tregs)2 and by the release of type I IFNα/β and indoleamine 2,3-dioxygenase.3,4 In an elegant article, a team led by Zhu et al identified that expression and release of VIP by donor pDCs regulated alloreactive T-cell activation and GVHD.1 VIP is an evolutionary highly conserved 28-amino-acid peptide. VIP functions as a neuromodulator and neurotransmitter by binding to VPAC1 and VPAC2 class II G protein-coupled receptors. Strikingly, VIP fulfills a plethora of physiological functions ranging from vasodilation, smooth muscle contraction and relaxation, and inhibition of gastric acid secretion to modulating epithelial paracellular permeability. Notably, VIP alsoproved to be an anti-inflammatory molecule by regulating Treg release of IL-10 and transforming growth factor β (TGFβ) and promoting Th2 polarization over Th1 differentiation.5
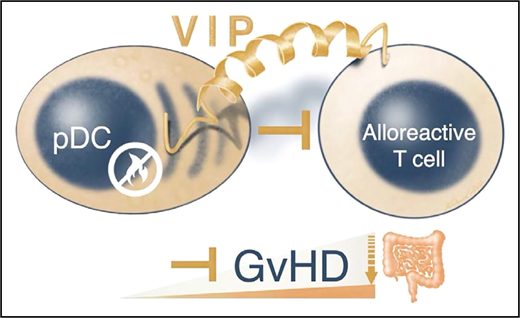
Zhu and colleagues investigated how donor pDCs regulate alloreactive donor T cells. They discovered that donor pDC-derived VIP is important for the control of GVHD (see figure). First, they found that human peripheral blood pDCs expressed VIP levels similar to those of mouse bone marrow pDCs. Surprisingly, VIP knockout (VIP-KO) mice showed increased pDC numbers compared with pDC numbers in wild-type mouse marrow. Second, VIP-deficient pDCs were unable to suppress CD4+ and CD8+ T-cell proliferation in vitro, which resulted in higher activated IFN-γ+, ICOS +, and IFN-γ+:tumor necrosis factor-alpha (TNF-α+) T-cell ratios. Third, recipients receiving VIP-deficient pDCs instead of wild-type pDCs developed early intestinal hyperacute GVHD in the small and large bowel, which was usually lethal. Intriguingly, imaging revealed that donor pDCs followed migration patterns similar to those of donor T cells that initially home to secondary lymphoid tissues.6 This may explain why the research team observed that the immunomodulatory effects of pDCs were transitory and that pDCs predominantly control the initiation of intestinal aGVHD. Limited pDC numbers in GVHD target organs diminished their capacity to control GVHD during the effector phase. Consistent with the severe aGVHD phenotype, VIP-KO pDC recipients’ alloreactive donor T cells expanded vigorously and Treg numbers were reduced compared with those of VIP wild-type pDC recipients. The higher inflammatory cytokine levels in VIP-KO pDC recipients indicates that donor pDCs limit (via VIP) the expansion of granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing alloreactive donor T cells that are pathognomonic for intestinal GVHD.7 Accordingly, these uninhibited donor T cells exhibited strong inflammatory gene signatures of Fasn, Cyclophilin A, and the GM-CSF regulating transcription factor Bhlhe40. Remarkably, although donor pDCs preferentially homed to lympho-hematopoietic tissues, VIP production by donor pDCs did not abrogate graft-versus-leukemia activity of donor T cells in 2 different mouse models.
Donor pDCs can limit alloreactive T cell responses during the initiation of aGVHD. Zhu et al report that donor pDCs control intestinal GVHD via the tiny yet versatile 28-amino-acid VIP.
Donor pDCs can limit alloreactive T cell responses during the initiation of aGVHD. Zhu et al report that donor pDCs control intestinal GVHD via the tiny yet versatile 28-amino-acid VIP.
Considering the versatile nature of VIP, it is unlikely that a single molecular pathway will explain all of the impact of VIP on GVHD. The article by Zhu et al identifies VIP as a key controller of alloreactive T cells. Clearly, this proof-of concept article will also stimulate clinical trials that investigate whether donor pDCs and/or treatment with VIP can protect patients from intestinal aGVHD.
Conflict-of-interest disclosure: A.B. is a scientific cofounder of Aamuthera Biotech GmbH and Dualyx NV. The remaining author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal