In this issue of Blood, Ferrero et al1 present a comprehensive, longitudinal analysis of molecular measurable residual disease (MRD) assessments in patients with mantle cell lymphoma (MCL) treated with chemoimmunotherapy, autologous stem cell transplant (ASCT), and maintenance lenalidomide. This work provides further insights into the variability observed in patient outcome in MCL, while emphasizing that the predictive power of MRD is improved when it is used longitudinally across the disease course.
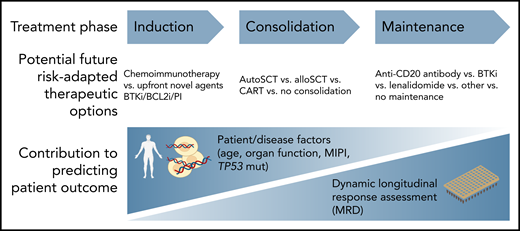
To provide effective personalized therapy for patients with hematological malignancy, an understanding of the likely outcomes after any given treatment regimen is necessary. Accurately predicting outcomes associated with various treatment regimens allows the physician and patient to make an informed choice together about which therapeutic path to pursue. In MCL (and indeed almost all hematological malignancy), baseline patient and disease factors (eg, age, organ function, MIPI[Mantle Cell Lymphoma International Prognostic Index], TP53 mutation status) have traditionally been studied to account for the variability in outcome associated with a given therapeutic pathway. However, across almost all hematological malignancies studied, response to therapy as measured by sensitive MRD methodologies consistently outperforms assessment of baseline variables in predicting patient outcome (see figure).
MRD for predicting outcome in MCL. AlloSCT, allogeneic stem celltransplant; autoSCT, autologous stem cell transplant; BCL2i, BCL2 inhibitor; BTKi, Bruton tyrosine kinase inhibitor; CART, chimeric antigen receptor T cell; PI, proteasome inhibitor.
MRD for predicting outcome in MCL. AlloSCT, allogeneic stem celltransplant; autoSCT, autologous stem cell transplant; BCL2i, BCL2 inhibitor; BTKi, Bruton tyrosine kinase inhibitor; CART, chimeric antigen receptor T cell; PI, proteasome inhibitor.
In their work, Ferrero et al assessed MRD across a treatment regimen consisting of chemoimmunotherapy followed by ASCT and a randomization to either 24 months of posttransplant lenalidomide or observation. Their study paints a detailed picture of response kinetics and predictive value of MRD in MCL. Although bone marrow MRD assessment at 6 months after ASCT was predictive of relapse risk, integrating longitudinal data and dynamic changes in MRD status over time enabled the authors to predict disease progression with increased certainty. When considering longitudinal follow-up, the presence of persistent MRD positivity or alternating positive/negative MRD was a more powerful predictor of disease progression. Conversely, the presence of multiple negative MRD results correlated with reduced relapse risk.
To derive these predictive timepoints, Ferrero et al used a well-established suite of molecular MRD techniques (quantitative real-time polymerase chain reaction [RT-qPCR] and nested qualitative PCR) within the standardized framework of the EuroMRD group. This approach involves the design and validation of patient-specific primers/assays targeting either immunoglobulin heavy chain (IGH)-CCND1 or the unique IGH clonotype. Using this approach, the authors were able to identify a trackable molecular marker in 250/300 (83%) patients (and 184/225 [82%] patients with adequate samples where assays could be designed with an acceptable standard curve for RT-qPCR). Although this type of conventional allele-specific PCR is an established approach, next-generation sequencing (NGS) is increasingly being used to detect patient-specific clonotypes across lymphoid malignancy with the advantage of a single assay being able to be used for all patients. Indeed, NGS-based MRD assessment has been used in MCL previously in the context of bendamustine-rituximab (± bortezomib) followed by rituximab consolidation (± lenalidomide) with only 2.7% of patients not having a trackable clone identified and 92% of trackable clones being able to be identified from peripheral blood/bone marrow aspirate samples.2 Other technological advances that may improve the predictive value of MRD in MCL include the use of circulating tumor DNA as well as using multigene panel MRD approaches, such as CAPP-Seq/PhasED-seq,3 which may give additional relevant genomic information about the nature of resistant clones.
In the primary publication of the trial associated with the work by Ferrero et al, after a median follow-up of 38 months, the use of lenalidomide after ASCT resulted in an improvement in progression-free survival (PFS) compared with placebo (3-year PFS 80% vs 64%, P = .012; hazard ratio, 0.51).4 Although overall survival (OS) was comparable between the 2 arms, the study was neither designed nor powered for this purpose. Lenalidomide was associated with substantially more toxicity, including grade ≥3 hematologic infection and secondary malignancy. For an incurable malignancy such as MCL, any maintenance therapy that results in PFS benefit without improvements in OS must be considered carefully. This underscores the relevance of the associated MRD study to predict which patients may derive the most benefit from continuing potentially toxic therapy and alternatively those that may be able to cease therapy without compromising disease control.
In the LYMA trial, in contrast, patients were treated with 4 cycles of rituximab-dexamethasone, cytarabine and cisplatin (R-DHAP) followed by ASCT, and responders were randomized to either 3 years of maintenance rituximab or observation.5 Maintenance rituximab resulted in superior OS compared with observation (4-year OS 88.7% vs 81.4%, P = .041) and did not result in substantial increases in toxicity compared with observation. Appropriate samples were available for MRD analysis in 220 patients from peripheral blood and/or bone marrow aspirate and assessed using a similar methodology to that used by Ferrero et al.6 MRD status after R-DHAP (pre-ASCT) predicted PFS and OS, and maintenance rituximab improved PFS and OS irrespective of MRD status.6 A similar patient-specific qualitative molecular MRD approach was built into the Nordic MCL2 study, in which patients treated with chemoimmunotherapy followed by ASCT underwent serial monitoring of peripheral blood and bone marrow aspirate.7 In this trial, 78 of the 145 patients who underwent ASCT had a molecular marker and 36 developed molecular relapse: 10 of whom had concurrent clinical relapse, and 26 of whom underwent preemptive treatment leading in reinduction of molecular remission in 92% of patients.8
It is clear from the work of Ferrero et al and others that assessment of molecular MRD is improving our ability to predict outcome in patients with MCL. However, robust data to support changes in treatment approaches will only be possible if researchers continue to prospectively test MRD-guided decision strategies as a part of trial design as well as prioritizing samples for these critical associated correlative studies. With the potential for further novel, widely applicable and sensitive molecular MRD technologies in the future as well as ongoing trials evaluating well-tolerated and effective agents such as Bruton tyrosine kinase inhibitors, MRD-guided therapy in MCL has the potential to allow deescalation/intensification of therapy to deliver better outcomes for patients.
Conflict-of-interest disclosure: P.B. consulted for, advised, or received honoraria from Adaptive Biotechnologies, AstraZeneca, and Servier. C.Y.C. consulted for, advised, or received honoraria from Roche, Janssen, MSD, Gilead, AstraZeneca, Lilly, TG Therapeutics, Beigene, Novartis, and BMS; and received research funding from BMS, Roche, Abbvie, and MSD.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal