In this issue of Blood, Huber et al1 present results of an important prospective clinical trial, demonstrating that the combination of obinutuzumab, ibrutinib, and venetoclax produces high rates of undetectable measurable residual disease and remarkable 2-year progression-free survival in previously untreated del(17p) or TP53-mutated chronic lymphocytic leukemia (CLL).
Cancer cells evolve resistance mechanisms to evade successive generations of cancer treatments. In CLL, targeted agents such as B-cell receptor signaling inhibitors (BCRi; eg, ibrutinib) and BCL-2 inhibitors (BCL2i; eg, venetoclax) are rightly hailed as contemporary paradigm-changing treatments. However, CLL eradication is uncommon with ibrutinib, and, not infrequently, patients relapse from single-agent ibrutinib or venetoclax after remissions of variable duration.
In contrast to chemotherapy-based treatments wherein del(17p)/TP53-deleted or TP53-mutated CLL cells are ubiquitously selected, preferential selection for TP53-defective CLL subclones is not generally seen with resistance to targeted therapy. Instead, acquired BTK/PLCG2 or BCL2 mutations that disrupt drug binding or circumvent the drug target are frequently responsible for resistance to ibrutinib and venetoclax, respectively. Within an individual, multiple CLL subclones often simultaneously coevolve different BTK/PLCG2 or BCL2 mutations.2,3 Additionally, other diverse mechanisms have been reported including the loss of extrinsic apoptotic TNF-related apoptosis-inducing ligand receptors in ibrutinib resistance,4 as well as upregulation of the antiapoptotic protein MCL1 and enhanced capacity for oxidative phosphorylation in venetoclax resistance.5
Given the lack of preferential drug-induced selection for del(17p)/TP53-mutant cells, one might expect ibrutinib or venetoclax to be equally efficacious in this traditionally poor-risk CLL subgroup. Indeed, response rates to ibrutinib and venetoclax are similar in TP53 wild-type and del(17p)/TP53-mutated CLL. Nevertheless, long-term survival outcomes remain inferior in the del(17p)/TP53-mutated subgroup.6,7 A likely explanation lies in the pivotal role of p53 in the maintenance of genome integrity, with p53 functional loss resulting in genomic instability that provides a permissive state for the acquisition of resistance mechanisms. Alternatively, TP53 defects may simply be surrogates for clonal complexity arising from heterogeneous genetic lesions. In any case, attaining measurable residual disease (MRD) negativity may have particular relevance in TP53-defective CLL with its inherent propensity to evolve resistance mechanisms. A smaller pool of residual CLL cells from which resistance could develop results potentially in less chance of relapse. There is therefore rationale for offering the best possible CLL treatment up front to achieve the most profound remission.
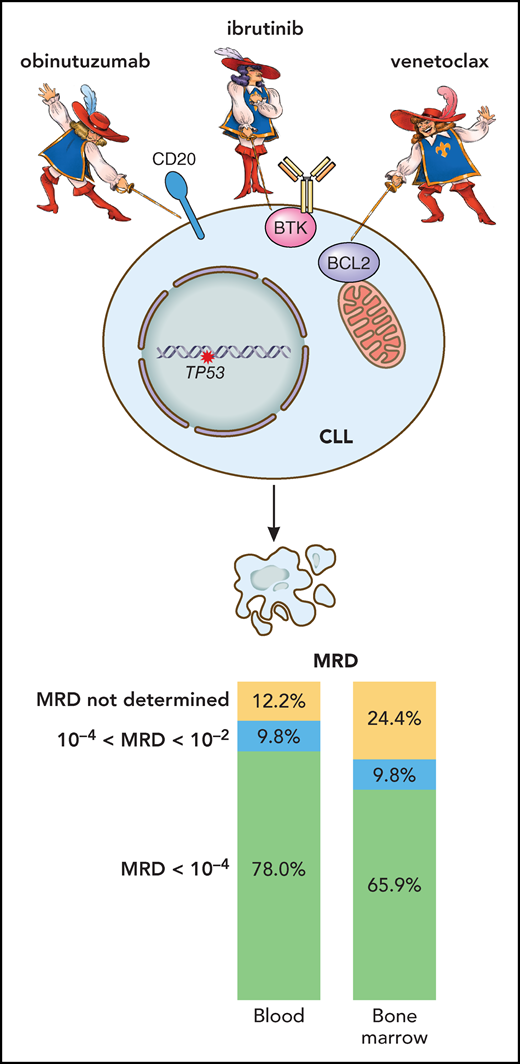
In this regard, Huber et al show in treatment-naïve patients with del(17p)/TP53-mutated CLL that combining ibrutinib, venetoclax, and the anti-CD20 monoclonal antibody obinutuzumab is generally well tolerated and yields enviable MRD-negative (<10−4) response rates of 78% in the peripheral blood (PB) and 66% in the bone marrow (BM) after 15 months of treatment that involved all 3 agents in the initial 6 months (induction), ibrutinib plus venetoclax in the next 6 months (consolidation), and ibrutinib alone thereafter (maintenance) (see figure). This translates into remarkable 2-year progression-free survival (PFS) and overall survival of 95%. These results for triplet therapy appear to compare favorably with the frontline venetoclax plus obinutuzumab data from the CLL14 trial showing 68% PB MRD-negative rate and ∼70% 2-year PFS in del(17p)/TP53-mutated CLL8 and represent a major improvement on the historic ∼20% to 30% 2-year PFS with frontline chemoimmunotherapy. Conversely, recent frontline data from the CAPTIVATE study demonstrate a similar BM MRD-negative rate of 66% with dual ibrutinib and venetoclax in del(17p)/TP53-mutated CLL.9 Whether triplet therapy is indeed superior to treatment with dual- or single-targeted agents needs to be determined within prospective randomized studies.
Results of the CLL2-GIVe trial in patients with previously untreated CLL harboring TP53 alterations. The combined targeting of CD20 (obinutuzumab), BTK (ibrutinib), and BCL-2 (venetoclax) leads to an impressive reduction in CLL tumor load, demonstrated by undetectable MRD in PB and BM in a high proportion of treated patients. Professional illustration by Patrick Lane, ScEYEnce Studios.
Results of the CLL2-GIVe trial in patients with previously untreated CLL harboring TP53 alterations. The combined targeting of CD20 (obinutuzumab), BTK (ibrutinib), and BCL-2 (venetoclax) leads to an impressive reduction in CLL tumor load, demonstrated by undetectable MRD in PB and BM in a high proportion of treated patients. Professional illustration by Patrick Lane, ScEYEnce Studios.
In this MRD-adaptive trial of Huber et al, treatment was stopped once complete response (as per International Workshop on Chronic Lymphocytic Leukemia criteria) with undetectable PB and BM MRD had been achieved within the consolidation or maintenance phase.1 Notwithstanding the excellent 2-year PFS, multiple relapses were observed at 24 to 34 months shortly after stopping treatment. This begs the question of whether 10−4 CLL cells is a sufficiently low MRD threshold to allow treatment cessation in del(17p)/TP53-mutated CLL with heightened genomic instability and clinical aggressiveness. Further modeling in large patient cohorts may help establish the likely depth of response required for sustained treatment-free remission in this CLL subgroup, and determine whether lengthening the duration of the consolidation and/or maintenance phase by a defined period beyond MRD negativity (<10−4) or offering continuous maintenance treatment will minimize CLL relapse. In this respect, rapid MRD depletion during the first 2 months of venetoclax-based treatment may be predictive of durable treatment-free remission.10 Moreover, longitudinal monitoring of MRD and clonal evolution (eg, emergence of BTK/PLCG2 or BCL2 mutations) after treatment cessation may help preempt the need to restart treatment and guide the choice of salvage therapy.
It remains to be seen also whether treatment combinations incorporating second-generation BCRi or BCL2i will further improve MRD-negative response rates and survival outcomes in del(17p)/TP53-mutated CLL. Future innovations may lie in therapeutic approaches that embody the concept of evolutionary herding. In the context of del(17p)/TP53-mutated CLL, such approaches could involve combining therapies that target common CLL dependencies (eg, BCRi and BCL2i) with therapies that target dependencies specific to genomically unstable del(17p)/TP53-mutated subclones. The former could eliminate the majority of CLL cells, whereas the latter offers the prospect of suppressing clonal evolution and hence averting leukemia relapse. Experimental TP53-targeting strategies in CLL include inhibition of the p53-antagonist MDM2 to upregulate wild-type p53, restoration of p53-associated tumor suppressive function in TP53-mutant cells, and induction of synthetic lethality by exploiting unique cellular vulnerabilities conferred by p53 loss. In addition, the immunogenicity of TP53 alterations and the interactions between p53 defects and tumor immunity constitute compelling therapeutic targets for future investigation.
These novel therapeutic strategies remain largely in preclinical development. Meanwhile, the findings of Huber et al undoubtedly represent a major clinical advance that is set to influence CLL management, providing a powerful new addition to our therapeutic armamentarium that can be readily deployed to improve the outlook of patients with del(17p)/TP53-mutated CLL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal