In this issue of Blood, Lewis et al1 highlight the importance of sphingolipid-regulating therapeutics by reporting a ceramide-mediated mechanism downregulating myeloid cell leukemia 1 (Mcl-1) to restore venetoclax sensitivity of acute myeloid leukemia (AML).
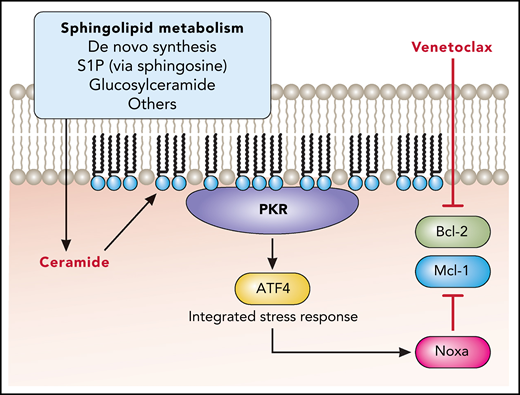
Here, Lewis et al build upon their earlier work demonstrating anti-AML efficacy for the sphingosine kinase inhibitor MP-A08.2 The prior study showed that MP-A08 could regulate Mcl-1. However, the mechanism remained elusive as well as whether anti-AML efficacy was due to the lack of sphingosine-1-phosphate or alternatively to the accumulation of ceramide. In the present study, Lewis et al find that ceramide specifically generated in response to sphingosine kinase inhibition binds and activates protein kinase R (see figure). The integrated stress response is subsequently stimulated through activation of the transcription factor 4 (ATF4), and this mediates the production of Noxa, an endogenous inhibitor of Mcl-1. Importantly, Lewis et al validate this ceramide-dependent effect using MP-A08 as well as an acid ceramidase inhibitor. Therefore, a major implication of this study is that the accumulation of ceramide is essential to initiate an anti-AML mechanism downregulating Mcl-1. Moreover, this highlights the utility of ceramide-elevating therapeutics as a potentially major advance in the treatment of AML and related hematologic disorders.
Ceramide-elevating therapy promotes protein kinase R (PKR) activation through direct binding to ceramide. This promotes the integrated stress response through phosphorylation of eukaryotic initiation factor 2 alpha, which activates the transcription factor ATF4. Subsequent expression of Noxa blocks antiapoptotic Mcl-1 to overcome resistance to the Bcl-2 inhibitor venetoclax. Professional illustration by Patrick Lane, ScEYEnce Studios.
Ceramide-elevating therapy promotes protein kinase R (PKR) activation through direct binding to ceramide. This promotes the integrated stress response through phosphorylation of eukaryotic initiation factor 2 alpha, which activates the transcription factor ATF4. Subsequent expression of Noxa blocks antiapoptotic Mcl-1 to overcome resistance to the Bcl-2 inhibitor venetoclax. Professional illustration by Patrick Lane, ScEYEnce Studios.
Sphingolipids are a major class of lipid that plays important roles as regulators of cell fate and function in addition to being key membrane components.3 The bioactive properties of sphingolipids are arguably some of their more interesting features, especially when considering the clinical relevance of sphingolipid-regulating therapeutics. Ceramide represents a major subtype of sphingolipid that is classically associated as a regulator of cell death and stress.3 It can be generated through a de novo synthetic pathway or through the catabolism of many other sphingolipids. Ceramide also serves as a precursor to many of these other sphingolipids. Therefore, sphingolipid metabolism can be understood through a ceramide-centric perspective. Moreover, therapeutics that augment cellular ceramide levels may do so either by promoting ceramide generation, blocking its metabolism, or by delivery of exogenous ceramide.
Sphingolipid-regulating therapeutics, or more specifically ceramide-elevating therapeutics, are poised as potentially important anticancer agents. Fundamentally, this is because elevations in ceramide are associated with cancer cell death.3 Several ceramide-elevating strategies have recently been reported to exert anti-AML efficacy. These include inhibitors of sphingosine kinases,1,2 acid ceramidase,4 as well as glucosylceramide synthase.5 In addition, delivery of ceramide has been evaluated using a water-soluble ceramide analog,6 as well as a nanoliposomal ceramide formulation.7 Intriguingly, nanoliposomal ceramide exerted potent efficacy toward AML arising out of myelodysplastic syndrome. It is important to note that although dysfunctional sphingolipid metabolism may be a unifying theme across many subtypes of AML, it is not the case that all ceramide metabolic outlets are upregulated in each AML case.7 For that reason, eventual clinical use of ceramide-elevating therapeutics may be situation-dependent and should be informed and adjusted by diagnostics that can follow these sphingolipid pathways.
Overcoming therapeutic resistance is another very important theme of Lewis et al. Venetoclax is a targeted therapeutic in the BH3 mimetic class, which targets the antiapoptotic B-cell lymphoma 2 (Bcl-2) protein.8 It is used in the treatment of various hematologic malignancies, including AML, in combination with low-dose cytarabine. Resistance to venetoclax, or Bcl-2 inhibition, is often due to upregulation of other antiapoptotic Bcl-2 family members.8 This includes Mcl-1, which has been the subject of additional drug discovery efforts. However, there may be limitations to the use of specific Mcl-1 inhibitors. The theoretical use of these inhibitors may be limited to short durations to avoid toxicity associated with the disruption of normal physiological roles for Mcl-1. There are also other pathways of resistance to Bcl-2 inhibition aside from upregulation of Mcl-1. This includes upregulation of mitogenic signaling pathways,8 many which are known to be regulated by ceramide, including the Akt and MEK/Erk pathways.3 Overall, Lewis et al’s characterization of Mcl-1 downregulation by ceramide contributes profoundly to our understanding of the oncogenic pathways effected by ceramide. Therefore, ceramide-elevating therapies may hold more promise, as they can broadly target and limit multiple pathways of Bcl-2 inhibitor resistance.
As intriguing as their other findings are, the recent findings of Lewis et al aided in identifying the integrated stress response as a key pathway linking ceramide to regulation of Mcl-1. This is noteworthy because it may represent a unique vulnerability in cells of hematopoietic origin. Specifically, the ATF4-mediated integrated stress response was previously described as an attribute of hematopoietic stem cells, including AML stem cells.9 This means that the ability of ceramide-elevating therapeutics to downregulate Mcl-1 may be possible owing to the prevalence of this integrated stress response in AML stem cells. Furthermore, the ability to impact the AML stem cell population means that ceramide-elevating therapeutics may be advantageously positioned to eradicate this key cellular population. Lewis et al showed that this leukemia-initiating population was disrupted by treatment with MP-A08, which was an effect also observed with other ceramide-elevating therapeutics.7
There are potential limitations to the utility of ceramide-elevating therapeutics. The first potential limitation is adaptive resistance owing to changes in ceramide metabolism, or more specifically, owing to upregulation of alternative ceramide metabolic outlets.3,7 To manage this, it is important to understand how the molecular evolution in AML can manifest in changes to ceramide metabolism. This may help identify links between AML molecular subtypes and ceramide metabolic pathways, which could aid the clinical selection of specific ceramide-elevating therapeutics. Moreover, therapeutics simultaneously targeting multiple ceramide metabolic outlets may be useful to avoid adaptive resistance.7 Last, it is imperative to define potentially harmful effects of ceramide-elevating therapeutics. A recent study linking disruption of sphingomyelin synthesis to the development of thrombocytopenia is noteworthy.10 Specifically, it was observed that the lack of sphingomyelin, and not an elevation in ceramide, promoted thrombocytopenia. That effect was uncovered using transgenic knockout mice but could extrapolate to potential sphingomyelin synthase inhibitors, which also are ceramide-elevating therapeutics.
Overall, the study by Lewis et al has advanced our understanding of ceramide-elevating AML therapy by defining a mechanism downregulating Mcl-1 to overcome Bcl-2 inhibitor resistance. Ultimately, this rationalizes improvement to standard care AML therapy with agents such as venetoclax through combination with ceramide-elevating therapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal