In this issue of Blood, Gauthier et al1 demonstrate that different chimeric antigen receptor (CAR) T-cell products independently associate with toxicity and efficacy outcomes in patients with relapsed or refractory large B-cell lymphoma (LBCL).
Three autologous anti-CD19 CAR T-cell products are currently approved by the US Food and Drug Administration (FDA) as third-line treatment and beyond for patients with relapsed or refractory LBCL: axicabtagene ciloleucel (axicel), tisagenlecleucel (tisacel), and lisocabtagene maraleucel (lisocel). In the 3 registration studies that resulted in their approval, ZUMA-1, JULIET, and TRANSCEND, the complete response (CR) rate was 58%, 40%, and 59%, respectively; the rate of grade ≥3 cytokine release syndrome (CRS) was 13%, 22%, and 1%, respectively; and the rate of grade ≥3 immune effector cell–associated neurotoxicity syndrome (ICANS) was 31%, 12%, and 13%, respectively.2-4 Of course, no interstudy comparison can be performed in regard to these toxicity and efficacy outcomes. In addition, there were significant differences in eligibility criteria and study design, such as the lack of bridging therapy in the ZUMA-1 study and the exclusion of primary mediastinal B-cell lymphoma from the JULIET study, to name just a few.
Because a randomized phase 2 or 3 study is very unlikely to ever happen, given the currently high financial stakes, only wisely designed retrospective studies can inform decision-making for the practicing clinician who has 3 good options available. Therefore, novel and robust statistical comparative methodologies need to be investigated. By using a matching-adjusted indirect comparison (MAIC), Schuster et al5 compared 106 patients from the JULIET study to 256 patients from the TRANSCEND study and observed higher response rates but comparable CR rates for patients treated with lisocel. No toxicity outcomes were assessed in this comparative analysis. Cartron et al6 have performed a very similar analysis, using summary level (rather than individual patient level) data from the JULIET study, and in addition to superior efficacy, they also observed inferior toxicity (in terms of CRS and persistent cytopenia) for lisocel as compared with tisacel.
The study by Gauthier et al used a directed diacyclic graph to account for confounding factors in a nonrandomized observational setting and compared 68 patients treated with commercial axicel to 31 patients treated with commercial tisacel and 30 patients treated with JCAR014. The latter has the same CAR design as lisocel (JCAR017) but significant differences in dosing, cell formulation, and manufacturing process. In this analysis, use of axicel independently associated with higher efficacy measured as CR rate, but also higher toxicity measured as CRS and ICANS rate. Of note, the comparison with tisacel was limited by sample size and statistical power. This study has some major limitations that greatly affect its applicability to clinical practice and emphasize the need for confirmation in larger datasets, such as small sample size, comparison of real-world to clinical trial setting, retrospective toxicity grading, and use of preleukapheresis (rather than preinfusion) clinical variables. Still, the study remains provocative and hypothesis-generating.
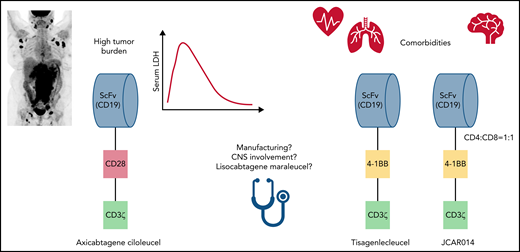
Data from the Gauthier et al study support the already common practice of avoiding the use of axicel in older and frail patients who may be at higher risk for toxicity-related mortality (see figure). It is important to note, however, that real-world data have not shown significantly worse outcomes for these patients, and novel strategies aimed at mitigating toxicity, especially ICANS, associated with the use of axicel are on the horizon.7 This study also supports the use of axicel for patients with more aggressive disease, as high lactate dehydrogenase and largest diameter size associated with a lower chance of CR (see figure). The latter 2 represent surrogate markers for tumor burden, one of the strongest predictors of response to CAR T-cell therapy.8 It is important to highlight that, despite these encouraging data, even the activity observed with axicel is suboptimal for patients with high tumor burden, and experimental combination strategies are under development for this very high-risk population.
Factors for CD19 CAR T-cell product selection in large B-cell lymphoma. CNS, central nervous system; LDH, lactate dehydrogenase; ScFv, single-chain variable fragment.
Factors for CD19 CAR T-cell product selection in large B-cell lymphoma. CNS, central nervous system; LDH, lactate dehydrogenase; ScFv, single-chain variable fragment.
Yet practicing clinicians sometimes have only limited options not addressed in this study (see figure). The manufacturing time for axicel is about 17 days, but it can be 30 days or longer for tisacel and lisocel, which frequently determines product selection that favors axicel, independently of safety concerns. Although it was not included in the FDA label, patients with active secondary central nervous system involvement are sometimes treated with CAR T-cell therapy in real-world practice, and encouraging efficacy data are currently available mainly for tisacel.9 Finally, conclusions regarding the comparative safety and efficacy of lisocel cannot be extrapolated from this analysis, despite the potential allusion and the similarities with JCAR014.
Although it is tempting to think that the costimulatory domain (CD28 for axicel and 4-1BB for tisacel and JCAR014) may be solely responsible for the superior efficacy and toxicity data reported for axicel in this study, it is also important to acknowledge the heterogeneity of the infusion product and its potential impact. These include a central memory T-cell phenotype associated with better efficacy and the presence of a monocyte-like population associated with a higher risk of ICANS.10 Ongoing research aimed at manipulating and better controlling the composition of the infusion product may help overcome some of the limitations observed in this study, and increase the number of available options for the practicing clinician in the near future.
Conflict-of-interest disclosure: P.S. served on advisory boards and/or was a consultant for Genentech/Roche, Hutchinson MediPharma, TG Therapeutics, and ADC Therapeutics and has received research support from AstraZeneca, Acerta, and ALX Oncology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal