In this issue of Blood, Termini et al have identified syndecan-2 as a new cell surface marker that can enrich functional hematopoietic stem cells (HSCs) in mice, and regulate HSC quiescence through Cdkn1c (p57).1
HSCs are the key components of bone marrow transplantation, the renowned therapy that has been successfully used to treat many blood and immune diseases and that possesses the immense potential to treat many more.2,3 HSCs are rare. Identifying them from other cells in the bone marrow is essential for evaluating the donor cell population and for discovering their regulatory mechanism. Understanding how HSCs are regulated can help improve bone marrow transplantation and develop new treatment strategies independent of transplantation.
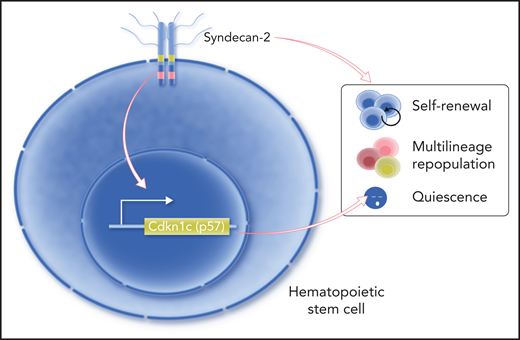
Over the past decades, several HSC markers have been identified, most of which have no regulatory role in HSCs.2,4-6 Here, Termini et al have identified syndecan-2, a heparan sulfate proteoglycan, as a novel marker for identifying and isolating mouse HSCs. Furthermore, they have revealed its roles in regulating mouse HSC functions during bone marrow transplantation (see figure).
Termini et al showed that syndecan-2 is expressed on the cell surface of HSCs that exhibit enhanced self-renewal and blood repopulation capacities. Their study also suggests that syndecan-2 regulates HSC quiescence through modulating the expression of Cdkn1c (p57). Professional illustration by Somersault18:24.
Termini et al showed that syndecan-2 is expressed on the cell surface of HSCs that exhibit enhanced self-renewal and blood repopulation capacities. Their study also suggests that syndecan-2 regulates HSC quiescence through modulating the expression of Cdkn1c (p57). Professional illustration by Somersault18:24.
Based on previous transcriptome studies, Termini et al identified and verified that the expression of syndecan-2 is elevated in phenotypic HSCs compared with other hematopoietic progenitor cells including multipotent progenitor cells. They also showed that this unique expression pattern holds true in human HSCs and hematopoietic progenitor cells. In addition, it is not displayed by other syndecan family proteins such as syndecan-1, syndecan-3, and syndecan-4. More importantly, using competitive transplantation assays, Termini et al showed that syndecan-2 can be used to sort for functional HSCs with enhanced self-renewal and differentiation capacities: (1) syndecan-2 alone was significantly better than CD150 alone for enriching functional HSCs from lineage negative bone marrow cells; (2) syndecan-2+CD34−KSL cells exhibited better long-term multilineage blood repopulation and increased contribution to phenotypic HSCs compared with syndecan-2−CD34−KSL cells and CD34−KSL cells in both primary and secondary recipients; (3) a limiting dilution assay revealed that the functional HSC frequency of syndecan-2+CD34−KSL cells is comparable to that of CD150+CD34−KSL cells, which is 17-fold higher compared with syndecan-2−CD34−KSL cells; and (4) mice transplanted with syndecan2+CD150+CD34−KSL cells exhibited 15-fold increased multilineage hematopoietic repopulation compared with mice transplanted with syndecan-2−CD150+CD34−KSL cells. These data demonstrated that syndecan-2 marks functional HSCs with comparable efficiency as other commonly used HSC markers such as CD150.6 Furthermore, nearly all of the in vivo repopulating capacity of CD150+CD34−KSL cells was contained within the syndecan-2+CD150+CD34−KSL cell population, suggesting that syndecan-2 can resolve the heterogeneity of the CD150+CD34−KSL cell population. The study from Termini et al adds to the accumulating literature helping investigators to better identify and study HSCs.
In addition to the enhanced self-renewal and differentiation capacities, syndecan-2+CD34−KSL cells also exhibited increased frequency in the G0 phase and decreased frequency in the G1 phase compared with syndecan-2−CD34−KSL cells and CD34−KSL cells. Despite the quiescence of syndecan-2+CD34−KSL cells, these cells could enter the cell cycle and produce significantly more CD34−KSL cells in vitro compared with syndecan-2−CD34−KSL cells. In competitive primary and secondary transplantations, the progeny of cultured syndecan-2+CD34−KSL cells showed increased capacities for self-renewal and multilineage hematopoieticrepopulation compared with the progeny of syndecan-2−CD34−KSL cells and CD34−KSL cells. HSC quiescence is thought to underly their self-renewal and differentiation potentials as disturbing the quiescence would cause HSCs to lose their long-term blood repopulation capacity.7 Therefore, the findings of Termini et al suggest that syndecan-2 may regulate HSC self-renewal and differentiation through modulating their quiescence.
To determine the functional role of syndecan-2 in HSCs, Termini et al used lentiviral short hairpin RNAs to knock down syndecan-2 expression in HSCs and showed reduced myeloid repopulation in primary recipients and reduced multilineage repopulation in secondary recipients. Furthermore, syndecan-2 knockdown reduced the fraction of phenotypic HSCs in the G0 phase and suppressed the expression of Cdkn1c, whereas syndecan-2 overexpression produced the opposite effects. Downregulation of Cdkn1c in combination with downregulation of syndecan-2 did not further reduce HSC quiescence, suggesting that syndecan-2 regulates HSC quiescence through its control of Cdkn1c expression.
Nonetheless, it is worth noting that not all functional HSCs express syndecan-2. The limiting dilution transplantation results from Termini et al indicate that approximately one-third of functional HSCs do not express syndecan-2. Moreover, syndecan-2 expression in HSCs can be turned on and off in vitro, suggesting that syndecan-2 marks 2 interchangeable states of HSCs instead of 2 distinct subtypes of HSCs. However, 8 weeks after transplantation, donor syndecan-2+CD34−KSL cells gave rise to significantly more syndecan-2+CD34−KSL cells compared with donor syndecan-2−CD34−KSL cells. And donor syndecan-2−CD34−KSL cells rarely produced any syndecan-2+CD34−KSL cells. Therefore, HSCs do not appear to freely alternate between the syndecan-2 positive and negative states. Further investigation on how HSCs switch between the syndecan-2+ and syndecan-2− states can provide more insights into the regulatory mechanism of HSC quiescence.
Syndecan-2 is a member of the syndecan family, consisting of 4 transmembrane heparan sulfate proteoglycans in mammals.8 Syndecans interact with other cell surface receptors, such as growth factor receptors and integrins, and play key roles in regulating many cellular behaviors and diseases.8 Inhibition of heparan sulfate synthesis and adding heparan sulfate mimetics can mobilize HSCs,9,10 suggesting the role of heparan sulfates in the retention of HSCs in their bone marrow niche. Therefore, the discovery of syndecan-2’s regulatory role in HSCs may lead to potential applications for improving HSC functions during bone marrow transplantation. Future investigation of syndecan-2 in human HSCs can elucidate its translational potential because syndecan-2 expression is also elevated in human HSCs as reported by Termini et al. However, the authors also showed that bone marrow cells from the syndecan-2 knockout mice exhibited modest decrease in hematopoietic repopulation after bone marrow transplantation. This suggests the existence of a compensatory mechanism that was absent in the acute knockdown of syndecan-2 as demonstrated by Termini et al. The study from Termini et al could lead to future investigations on the translational potential and regulatory mechanism of syndecan-2 in HSCs, which could help improve HSC-based cell and gene therapies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal