In this issue of Blood, Purroy et al1 use cutting-edge technology to demonstrate that the state of immune cells in chronic lymphocytic leukemia (CLL) is unequivocally different from that of immune cells in healthy individuals and is characterized by an increased number of mostly inhibitory cellular interactions. The monoclonal B-cell lymphocytosis (MBL)-to-CLL transition is associated with relative stability in the immune cell compartment, suggesting that immune dysfunction is an early event in CLL pathogenesis.
CLL is characterized by a numerically, phenotypically, and functionally aberrant immune cell compartment. Antibody production, the complement system and neutrophil, natural killer cell, and macrophage function are abnormal in CLL.2 T cells from patients with CLL undergo unbalanced polarization toward the immunosuppressive regulatory T (Treg) cell and Th2 > Th1 subsets. They also exhibit impaired immune synapse formation, deregulated cytotoxic function, poor adhesion, and poor migration capabilities.3 Immune system dysfunction hinders antitumor response and increases the risk for infectious complications, such that infections remain the main cause of death of patients with CLL, a public health problem exacerbated by the pandemic.4 Furthermore, MBL, a common premalignant condition with greater prevalence among older individuals, also confers an increased risk of infectious complications.5 This begs several questions, including: (1) at what point in disease development do these immune deficits arise; (2) can their evolution be detected as disease progresses from the precursor state to CLL, and (3) is correction of these deficits in CLL feasible? These are the questions addressed by Purroy et al. They conduct an single-cell RNA-Seq analysis of primary samples to first establish that there is little variability in distribution of immune cell subsets (T cells included) between nonprogressive MBL cases and CLL requiring therapy. Furthermore, analysis of serial samples from both disease categories revealed that little, if any, evolution occurs over the natural course of either condition. By contrast, there were clear distinctions in the composition of immune cell types in MBL/CLL vs in healthy individuals. Leukemic samples exhibited an increased proportion of CD8+ T cells, including T effector memory cells (a likely indication of high antigenic exposure in patients with CLL), as well as a higher number of differentially expressed genes compared with healthy donors. To further strengthen the point, Purroy et al analyzed paired samples from patients whose MBL progressed to CLL, confirming that such a transition is not accompanied by major compositional, phenotypic or transcriptional changes in immune cell subsets.
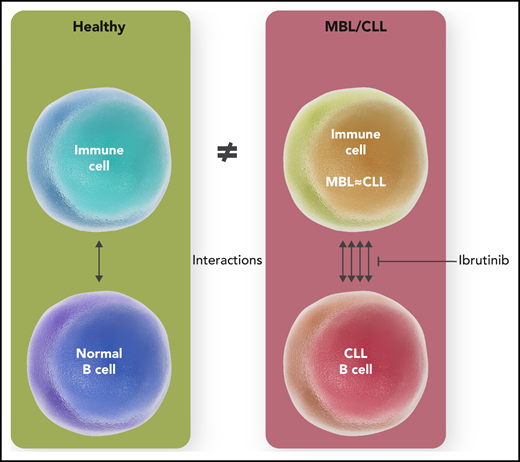
Surprising as this may be, particularly in context of the propensity for clonal evolution of the leukemic cell itself, well documented by the same group,6 this finding nevertheless suggests that immune deficits likely develop well before a patient with CLL gets their first treatment prescribed. Using sophisticated analytical software, this study reveals that, in contrast to healthy controls, cellular interactions abound in MBL/CLL with abundant cross talk between many ligand-receptor pairs (see figure), and with particular involvement of the myeloid cell lineage, an poorly studied underdog of the CLL microenvironment. The observation that inhibitory immune signals dominate in MBL, as in CLL, highlights that MBL is not a benign condition after all, as far as infectious complications are concerned.
Immune cells in MBL and CLL, although similar phenotypically and transcriptionally, demonstrate an increased number of (predominantly immunosuppressive) cellular interactions vis-a-vis the healthy donors that may be reversed by ibrutinib therapy. Professional illustration by Somersault18:24.
Immune cells in MBL and CLL, although similar phenotypically and transcriptionally, demonstrate an increased number of (predominantly immunosuppressive) cellular interactions vis-a-vis the healthy donors that may be reversed by ibrutinib therapy. Professional illustration by Somersault18:24.
The novel findings also establish new research directions. In this work, immune cell analysis was conducted using peripheral blood mononuclear cells. However, CLL progression may occur in the lymph node compartment. By focusing on a single compartment, it is possible that significant immunologic events went unnoticed. Future work should explore multiple compartments to fully understand how immune system dysfunction evolves in CLL. A small sample size, because of costs of high-end technology, is another factor that may have limited the authors' ability to document lower frequency events. This may explain why they failed to observe an increase in the Tregs subset in their samples, a rather consistent finding in CLL. A larger study might not only help confirm current findings but also reveal novel mechanisms underlying immunosuppression in CLL.
Can something be done to correct the immune deficit in B-cell neoplasia? Although chemoimmunotherapy results in unequivocal immune suppression in CLL, novel targeted agents exert complex effects.7 Inhibitors of Bruton tyrosine kinase (BTK; eg, ibrutinib) may reduce T-cell exhaustion, modulate T-cell polarization (toward the Th1 subset), and downregulate secretion of interleukin 10 by the CLL cells, thereby relieving undue pressure.8 Purroy et al find that treatment with ibrutinib “normalizes” cellular interactions in CLL, thus potentially boosting the immune cell compartment. It is, however, too early to recommend use of BTK inhibitors as immune modulators in CLL. In the CLL12 study that randomly assigned patients with treatment-naïve CLL to either ibrutinib or placebo, the frequencies of all-grade and severe infections were similar between the 2 arms.9 An intervention to boost immunity in MBL/CLL needs to be carefully balanced against the risks of adverse events, which in the case of BTK inhibitors include atrial fibrillation and bleeding.
In sum, this study sheds light on the pathogenesis of immune deregulation in CLL, suggesting that it may arise early in the disease development, and prompts further inquiries regarding mitigation of consequences.
Conflict-of-interest disclosure: A.V.D. has received consulting fees from AstraZeneca, Abbvie, BeiGene, Genentech, TG Therapeutics, Bayer Oncology, and Pharmacyclics and has ongoing research funding from AstraZeneca, Takeda Oncology, Bayer Oncology, Genentech, SecuraBio, MEI, TG Therapeutics, and Bristol Myers Squibb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal