In this issue of Blood, Bednarski et al1 describe the results of a clinical trial of memory-like natural killer (ML NK) cells for children and adolescents/young adults (AYA) with acute myeloid leukemia (AML) relapsed after transplant. ML NK cells were generated from the hematopoietic cell transplant (HCT) donor and thus were immune compatible, yet allogeneic, to the patient, setting up an incredibly unique clinical environment in which to evaluate various aspects of allogeneic NK cell therapy. Serial assessments of blood and bone marrow from each patient over an extended clinical course using mass cytometry and single-cell RNA sequencing provide new insights into the biology of memory NK cells and their utility in AML therapy.
NK cells have long been an attractive cellular platform for AML therapy because of their potent graft-versus-leukemia effect in the allogeneic setting and low rates of graft-versus-host disease (GVHD). However, clinical responses have been mixed and antileukemic NK subsets difficult to determine or harness therapeutically. Recently, memory NK cells have been a subset of interest for harnessing antileukemic functions. Memory responses with unique functional and molecular characteristics have been defined following exposure of NK cells to specific haptens, viruses, or cytokines.2 Human NK cells exhibiting cytokine-induced memory-like differentiation (CIML−, also known as ML NK) in response to the cytokine cocktail of interleukin-12 (IL-12), IL-15, and IL-18 exhibit prolonged survival and enhanced effector functions in leukemia models.3,4 ML NK cells have been evaluated in several recent trials for treatment of AML. The first-in-human clinical trial of adoptively transferred allogeneic ML NK cells after lymphodepletion in patients with relapsed/refractory AML (#NCT01898793) tested a single infusion of ML NK cells generated from a related major histocompatibility complex–haploidentical donor followed by low-dose IL-2 for 2 weeks to support NK expansion and function.4 Seven of 15 evaluable patients achieved a complete remission (CR), purporting the use of allogeneic ML NK cells as salvage therapy for relapsed AML and “bridge” to curative transplant. Given that recipient T cells recovered after lymphodepletion and eliminated the allogeneic donor NK cells within weeks, ML NK cell persistence could not be evaluated in this setting. To evaluate ML NK cell long-term persistence, patients with relapsed AML who had undergone standard-of-care haploidentical HCT were treated with ML NK cells generated from the same HCT donor (#NCT02782546), thus administering allogeneic ML NK in an “immune compatible” clinical context that would remove allorejection as a variable in NK survival. This ML NK cell “donor lymphocyte infusion” (DLI) to augment haploidentical HCT was well tolerated and resulted in CR in >90% of patients at day 28.5 In a more formidable clinical setting, such as patients with AML who relapse after HCT, in which standard-of-care salvage chemotherapy followed by T-cell DLI exhibits poor outcomes, the authors tackle a significant unmet need to develop effective treatments for these patients. Thus, donor ML NK cell infusion following standard salvage chemotherapy and T-cell DLI was tested in the current report (see figure). The investigators addressed several key issues surrounding ML NK cell therapy: feasibility of product generation from different donor types, expansion and persistence in the allogeneic setting, and incidence of GVHD given the incorporation of T-cell–based DLI.
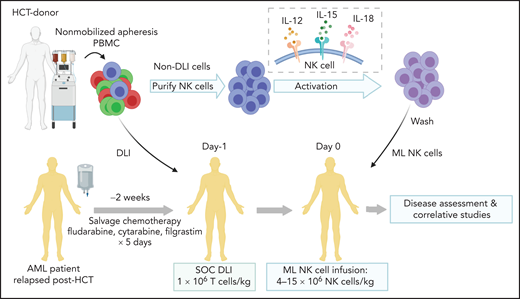
Overview of treatment schema. See Figure 1 in the article by Bednarski et al that begins on page 1670. PBMC, peripheral blood mononuclear cells; NK, natural killer; DLI, donor lymphocyte infusion; SOC, standard of care.
Overview of treatment schema. See Figure 1 in the article by Bednarski et al that begins on page 1670. PBMC, peripheral blood mononuclear cells; NK, natural killer; DLI, donor lymphocyte infusion; SOC, standard of care.
The authors showed that ML NK cells were successfully generated for all patients and from various HCT transplant donor types, including matched sibling, matched unrelated, and haploidentical. ML NK infusions were safe, and no patients experienced cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome. The overall treatment regimen (FLAG chemotherapy [fludarabine, cytarabine, and granulocyte colony-stimulating factor] plus T-cell DLI and ML NK infusion) was well tolerated, and only 1 patient who had mild skin GVHD at enrollment exhibited GVHD. At 1-month postinfusion, 4 of 9 patients exhibited CR, and 2 patients exhibited partial response. At day 100 postinfusion, 2 patients continued in CR. Importantly, in an immune-compatible environment (infused DLI T cells and ML NK cells were derived from the HCT donor), adoptively transferred ML NK cells persisted without administration of exogenous cytokines for at least 3 months in the majority of patients in both peripheral blood and bone marrow. Using a combination of mass cytometry and single-cell RNA sequencing, investigators showed that ML NK cells retained their distinct transcriptional signature after adoptive transfer. Finally, ML NK cells derived from patient blood or bone marrow exhibited antileukemic activity in correlative in vitro assays.
The findings of the current study inspire a few key points that deserve additional discussion. First, multidimensional phenotyping/genotyping of ML NK from patients revealed that the ML NK cells not only persisted in patients but also exhibited a preserved ML phenotype over time. These findings suggest for the first time in pediatric patients that the differentiation program of ML NK cells is maintained long term after transfer to patients and that, in an immune-compatible clinical environment, T-cell DLI could serve as a cytokine source to prolong survival and expand adoptively transferred ML NK cells. This is a key finding, as exogenous cytokine administration typically necessary for survival of adoptively transferred NK cells has been problematic (bioavailability, potency, toxicity, etc).6,7 Second, pediatric/AYA AML relapsed after HCT remains a significant clinical challenge. This patient population has low remission rates, high relapse rates, and standard/salvage treatment regimens with high toxicity, including chronic late effects even when cured.8,9 In the phase 1 trial reported here, treatment of patients with relapsed AML with donor ML NK cell therapy in combination with chemotherapy and DLI was well tolerated and had an overall response rate of 75% and CR rate of 50% at 1-month post-NK cell infusion, which compare favorably to outcomes with T-cell DLI (10% to 15%) or chemotherapy alone (40% to 70%).9
In an era when more allogeneic NK cell immunotherapies are being established, defining the safety, persistence, and efficacy of novel immunotherapy platforms such as ML NK in the allogeneic setting will be critical in advancing the field. Although applicability of ML NK cells to non-HCT or nonhematologic contexts remains to be tested, the approach detailed in the current report provides key clinical features of ML NK efficacy and safety in AML that are promising and warrant further investigation in larger cohorts.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal