Abstract
Background:
Acute myeloid leukemia (AML) is a clonal hematologic malignancy that generally affects older adults. Despite achieving complete remission (CR) in over two-third of patients with initial induction therapy and subsequent allogeneic hematopoietic stem cell transplantation in intermediate-high risk patients, more than half of AML patients experience disease relapse. The prognosis of patients with relapsed/refractory AML (RR-AML) is often poor and treatment modalities are limited. Chimeric antigen receptor T cell (CAR-T) therapy has shown promising results in lymphoid malignancies and myeloma, and these are now being explored for the management of RR-AML. In this systematic review and meta-analysis, we aimed to investigate the outcomes of CAR-T therapy in RR-AML patients.
Methods:
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a comprehensive literature search was conducted on three databases (PubMed, Cochrane Register of Controlled Trials, and Clinical trials.gov) using MeSH terms and keywords for "Leukemia, Myeloid, Acute" AND "Receptors, Chimeric Antigen" OR "adoptive immunotherapy" from the date of inception to April 2021. A total of 673 articles were screened and original studies reporting patients with RR-AML having CAR-T therapy as the only intervention were included while reviews, duplicate, and non-relevant articles were excluded. A total of 10 studies (8 clinical trials and 2 case reports) were included. The data for following outcomes were extracted: complete response (CR), partial response (PR), overall response rate (ORR), overall survival (OS), progression-free survival (PFS), stable disease (SD), progressive disease (PD), cytokine release syndrome (CRS) and neurotoxicity (NT). Quality evaluation was done using the NIH quality assessment tool. Inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with 95% confidence Interval (CI) were extracted to compute pooled analysis using the 'meta' package by Schwarzer et al. in the R programming language (version 4.16-2).
Results:
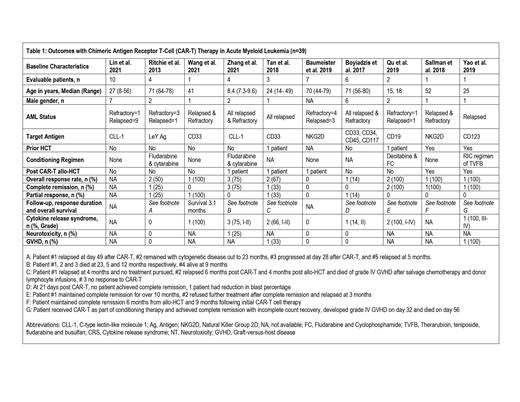
We identified 39 patients in 10 studies who received CAR-T therapy for RR-AML. Median age of patients was 35 (7.3-80) years and 59% (n=23) were male. The median follow-up time was 5 (0.7-23) months. (Table 1) Four patients had history of allogeneic hematopoietic stem cell transplant (HCT) prior to CAR-T therapy while subsequent HCT was performed in 5 patients. The pooled analysis showed a CR and ORR of 38.5% (95% CI 0.03-0.81, I 2 =66%, n=29) and 56% (CI 0.18-0.91, I 2=58%, n=29), respectively. Median duration of response was 5.5 (1-23) months. OS was reported from 1.9 months to 23 months. The pooled incidence of CRS and NT were 42.7% (95%CI 0.06-0.87, I 2=66%, n=28) and 1.3% (95% CI 0.00-0.16, I 2= 0%, n=21) respectively. Graft-versus-host disease (GVHD) was reported in 2 patients who had prior and subsequent HCT after CAR-T therapy; first patient developed grade IV GVHD in the setting of salvage therapy with donor lymphocyte infusions for relapsed disease 6 months' post CAR-T and 4 months post second allo-HCT while second patient received CAR-T as part of conditioning therapy and developed grade IV GVHD on day 32.
Conclusion:
CAR-T therapy has shown favorable results comparable to current salvage therapies for relapsed or refractory AML with an acceptable toxicity profile. However, there are several challenges including the heterogeneous biology of AML, lack of a targetable antigen expression on malignant cells, and immune escape and exhaustion. Future prospective studies with improved CAR-T constructs will hopefully improve the outcomes in this therapeutically challenging patient population.
Lin: AbbVie, Aptevo Therapeutics, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Novartis, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Abhyankar: Incyte/Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk: EcoR1 Capital: Consultancy; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding; Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding; Novartis: Research Funding; Astelllas Pharma: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Fresenius Biotech: Research Funding; Novartis: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal