Abstract
Background:
Autologous chimeric antigen receptor-T (CAR-T) cells that target BCMA (BCMA-CARs) have emerged as a promising treatment for multiple myeloma (MM). Current clinical protocols dictate that BCMA-CAR therapy is only used after patients have repeatedly relapsed. However, at this stage, the immunosuppressive nature of advanced MM and/or side-effects of the previous therapies cause T cell dysfunction and an unfavourable phenotype, such as exhaustion, senescence and loss of early memory cells.
An alternative and convenient pool of 'fitter' T cells are apheresis products that are routinely collected to obtain progenitor cells for autologous stem cell transplantation (ASCT), an intervention that is often carried out early in MM treatment. However, to mobilise the progenitor cells, patients are treated with G-CSF, which could have negative effects on T cells such as reduce proliferation, impair CD8 + T cell function and induce regulatory T cell (Treg) expansion. Whether this has an effect on the BCMA-CARs generated from these T cells, however, is unknown. Therefore, we aimed to establish whether G-CSF treatment had detrimental effects on T cell phenotype, and moreover, to ascertain whether BCMA-CARs that are generated from these T cells were impaired compared to those produced from T cells prior to G-CSF infusion.
Methods:
T cells were isolated from the blood of 9 patients with MM before and after 4 days of subcutaneous G-CSF administration (PRE G-CSF and POST G-CSF, respectively) prior to peripheral blood CD34 + cell harvesting for an ASCT as consolidation after first-line induction treatment. Following stimulation with anti-CD3/anti-CD28 beads and IL-2, T cells were transduced with ARI2h, an anti-BCMA CAR produced at our institution that is currently being explored in a clinical trial for relapsed/refractory MM (NCT04309981). Freshly-isolated T cells or expanded ARI2h cells were analysed by flow cytometry for markers of cell identity, activation, dysfunction and memory, or alternatively, challenged with an MM cell line (ARP-1 or U266) and then tested for cytokine production and cytotoxic ability. In addition, PRE and POST G-CSF ARI2h CARs were injected into ARP-1 tumour-bearing mice to assess their in vivo function.
Results:
Firstly, the phenotype of PRE G-CSF and POST G-CSF T cells, before CAR production, was analysed to identify effects of G-CSF treatment. Interestingly, there were fewer POST G-CSF CD8 + T cells with a stem cell memory (CCR7 +CD45RA +CD95 +) phenotype, but the proportion of naïve (CCR7 +CD45RA +CD95 -) cells and other memory populations was not significantly different. Moreover, POST G-CSF T cells had a lower CD4:CD8 ratio, but did not contain more senescent-like cells or display evidence of pre-activation or increased expression of exhaustion markers. Due to the known effect of G-CSF on CD4 + Treg expansion, the percentage of Tregs was also compared between the PRE G-CSF and POST G-CSF samples, but no difference was observed.
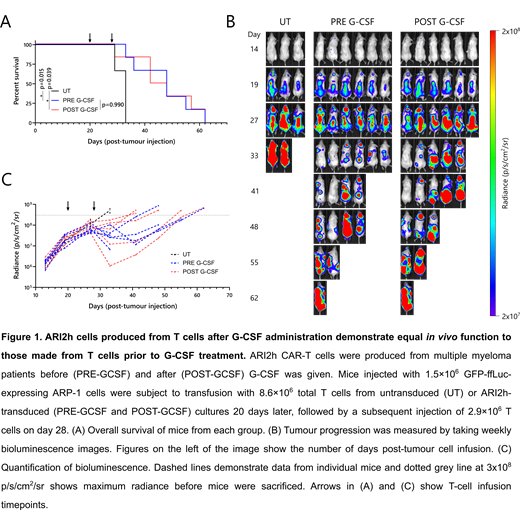
Following T-cell activation and CAR transduction, comparable transduction efficiencies and proliferation rates were obtained. Likewise, the in vitro function of PRE G-CSF and POST G-CSF ARI2h cells, as determined by assessing their cytotoxic response to MM cell lines and ability to produce effector molecules such as granzyme B, was similar. To test the in vivo function of ARI2h CAR-T cells expanded from PRE G-CSF and POST G-CSF samples, they were injected into a murine xenograft model of advanced MM. Mice administered with both PRE and POST G-CSF ARI2h cells survived longer than those given untransduced T cells (p=0.015 and p=0.039, respectively), but there was no difference in the longevity of mice between the PRE G-CSF and POST G-CSF groups (p=0.990) (Figure 1). The similarity of the in vitro and in vivo function of PRE and POST G-CSF ARI2h cells was reflected in the phenotype of the CAR-T cells after ex vivo expansion, with cells from both groups displaying equal levels of activation, exhaustion, and importantly for CAR-T cell activity, memory/effector phenotype.
Conclusions:
The in vitro and in vivo functions of ARI2h CAR-T cells when generated from either PRE G-CSF or POST G-CSF samples were comparable, despite G-CSF administration decreasing the CD8 + stem cell memory pool. Overall, we conclude that T cells from apheresis products, performed to collect G-CSF-mobilised peripheral blood progenitor cells for ASCT, are suitable for BCMA-CAR manufacture.
Lozano: Grifols: Honoraria; Terumo BCT: Honoraria, Research Funding; Macopharma: Research Funding. Fernandez de Larrea: BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; GSK: Honoraria; Sanofi: Consultancy; Janssen: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal