Abstract
Background: High Grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements (also known as Double-hit lymphoma (DHL)) is an aggressive B cell Non-Hodgkin lymphoma which has recently been recognized by the WHO as a distinct clinical entity from Diffuse Large B cell lymphoma (DLBCL). Compared to patients with DLBCL, patients with DHL have poor outcomes with standard R-CHOP chemoimmunotherapy, and the optimal treatment regimen for this high-risk lymphoma is unknown. Due to clinical and genetic overlap with Burkitt lymphoma (which shares translocations involving MYC), treatment of DHL with more intensive Burkitt lymphoma protocols is a common approach. However, these regimens are associated with significant toxicity, and the superiority of these regimens over standard R-CHOP remains uncertain. At our institution, R-CODOX-M/IVAC has been the preferred regimen for fit younger patients with DHL.
Objective: To review the outcomes of patients with DHL treated with R-CODOX-M/IVAC at our institution.
Methods: In this retrospective single-centre study, patients with DHL receiving R-CODOX-M/IVAC were identified by cross-referencing provincial cancer care data and hospital pharmacy data. All adult patients with a diagnosis of DHL and who were treated with R-CODOX-M/IVAC between January 2009 and January 2019 were included in the analysis. The primary outcomes is complete response (CR) per standard lymphoma response criteria. Secondary outcomes include median progression-free survival (PFS), overall survival at 1 year, and median overall survival. Kaplan-Meier analysis was used for survival analyses.
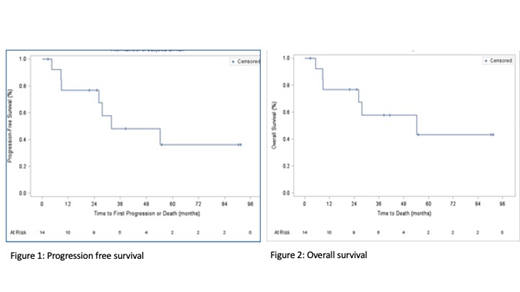
Results: 14 patients with DHL and meeting the inclusion criteria were identified and included in the analysis. The complete response rate was 35.7%. With a median followup time of 26 months, the median PFS was 31.8 months, and the median OS was 54.3 months. The overall survival at 1 year was 77%.
Conclusions: A previous meta-analysis demonstrated inferior outcomes for patients with DHL receiving RCHOP, with a median PFS of only 12.1 months. The optimal treatment of these patients remains to be defined. Dose-adjusted regimens have been associated with a significant improvement in PFS compared to RCHOP (21.6 months versus 12.1 months). Previous retrospective data suggests R-CODOX-M/IVAC results in more favourable outcomes 2 year PFS of 41% and 2 year OS of 53% (Sun et al., Clinical Lymphoma, Myeloma & Leukemia, 2015). Our data, although limited, that R-CODOX-M/IVAC may be associated with improved outcomes compared to published results with RCHOP. The limitations of this study include its retrospective design, lack of matched controls receiving RCHOP, and its small sample size. Nevertheless, this study adds to the small body of available evidence on this difficult to treat clinical entity.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal