Abstract
AVM0703 is a high concentration (24 mg/mL) dexamethasone phosphate drug product that permits the safe administration of the doses necessary to mobilize gamma delta+, bi-specific natural killer T (NKT) cells. AVM0703 is in clinical trials as a stand-alone treatment for relapsed/refractory (R/R) Non-Hodgkin's Lymphoma (NHL). Even more exciting is the potential combination of AVM0703 with standard chemotherapy to reduce the number of treatment cycles while maintaining efficacy. A therapeutic solution that reduces the number of chemotherapy cycles could lower the risk of secondary malignancies, decrease long-term toxicities, and limit costs associated with management of chemotherapy toxicities. AVM0703 has been well tolerated to date, with mild to moderate and self-limiting side effects. This indicates the use of AVM0703 in combination therapies may greatly improve quality of life for patients during treatment.
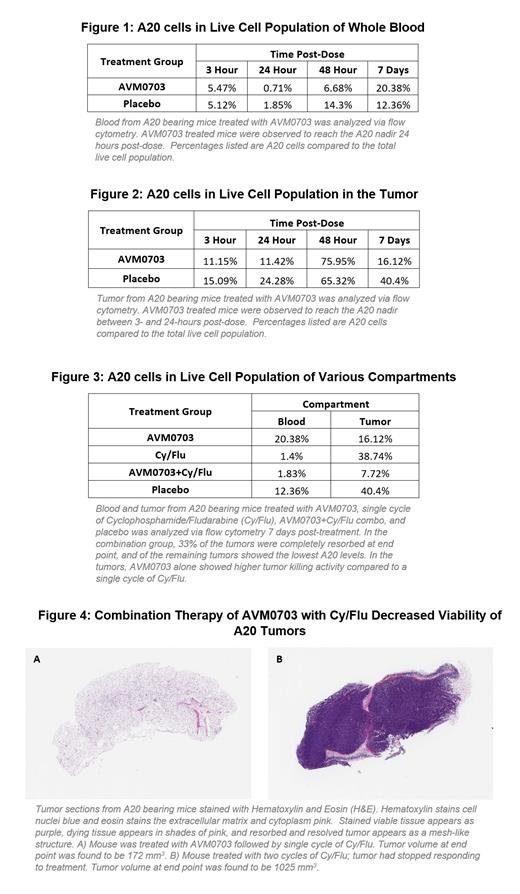
AVM0703 has been tested in an aggressive mouse B-cell lymphoma model (A20) alone and in combination with cyclophosphamide/fludarabine (CyFlu). The tumor killing effect of AVM0703 on A20 cells in Matrigel TM embedded tumors is evident as early as 3 hours after dosing, assessed by flow cytometry. Maximum A20 cell death is observed 24 hours after treatment in blood (Figure 1) and between 3- and 24-hours in tumors (Figure 2). Treatment with AVM0703 alone was compared with Cy/Flu alone and with a combination of single dose AVM0703 followed by one Cy/Flu cycle. Tumor growth was monitored by caliper measurements and blood and tumors were analyzed by flow cytometry at the end point, 7-days after Cy/Flu dosing. AVM0703 alone was superior to one cycle of Cy/Flu and the combination of a dose of AVM0703 followed by Cy/Flu was the most effective (Figure 3). Of the mice treated with the combination, 33% had no visible tumor 7-days post-dose. We are currently testing AVM0703 administered between 6- and 24-hours before a single Cy/Flu dose and predict that A20 eradication should be more complete in all compartments. Acute 7 day studies will be followed by long-term studies to evaluate A20 tumor escape or recurrence. In previous studies with long term monitoring of tumor growth, AVM0703 administered 3 days before one cycle of Cy/Flu induced long-term stable disease up to 50 days in tumor bearing mice (Figure 4a) compared to two cycles of Cy/Flu where 33% of the tumors escaped (Figure 4b). Additional studies in combination with R-CHOP are underway.
AVM0703 has the ability to debulk the tumor by mobilizing gamma delta+, bi-specific natural killer T (NKT) cells. When added to a chemotherapeutic regimen, the chemotherapy may be more effective due to the lower tumor burden in the body. As cancer survivors live longer, the late term consequences of chemotherapy have become apparent, which include secondary malignancies. The challenge for new treatments is to reduce toxicities without sacrificing efficacy. AVM0703 has the potential to be one solution when used in combination with chemotherapy. Future clinical trials will determine if debulking with AVM0703 results in a reduction of the number of necessary R-CHOP cycles while maintaining or enhancing the efficacy rate.
Deisher: AVM Biotechnology, LLC: Current Employment. Sawas: AVM Biotechnology, LLC: Current Employment. Suwito: AVM Biotechnology, LLC: Current Employment. Rylatt: AVM Biotechnology, LLC: Current Employment. Jarzyna: AVM Biotechnology, LLC: Current Employment. Zahid: AVM Biotechnology, LLC: Ended employment in the past 24 months. Parthasarathy: AVM Biotechnology, LLC: Consultancy, Ended employment in the past 24 months. Poulin: AVM Biotechnology, LLC: Current Employment. Lee-Diaz: AVM Biotechnology, LLC: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal