Abstract
Background
Tyrosine kinase inhibitors (TKIs) combined with intensive chemotherapy as a first-line treatment regimen significantly improved the prognosis of Philadelphia (Ph) chromosomal positive acute lymphoblastic leukemia (Ph+ALL). However, it has been reported that 74% of patients fail to complete 8 cycles of chemotherapy, and the early mortality rate during induction was as high as 13%. Our previous retrospective study determined an oral, chemo-free regimen (dasatinib plus prednisone) as induction and consolidation yields 100% of complete remission (CR) rates in Ph+ ALL with minimal induction death (Li XY, et al. Br J Haematol. 2020;189:e231-e234). To further verify the effectiveness of this completely oral and chemo-free regimen, we conducted a phase II, one-arm and multicenter trial (ChiCTR200038053).
Methods
Adults ≥16 years of age with new diagnosed Ph+ALL were eligible. Induction (Course I) used dasatinib 100 mg daily on days 1-28 and prednisone 1mg/kg/daily on days 1-21, 0.5 mg/kg/daily on days 22-28. Patients not in CR/CRi received Course I as second induction again. Two courses of consolidation (Course II and III) used dasatinib 100 mg daily on days 1-28 and prednisone 1mg/kg/daily on days 1-7. CNS prophylaxis used intrathecal cytarabine and dexamethasone. Patients 16-65 years old underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) if they had a HLA-matched donor; otherwise they received 6 cycles of hyper-CVAD chemotherapy alternating regimen-B (methotrexate, cytarabine) and regimen-A (cyclophosphamide, doxorubicin, vincristine, dexamethasone). The dose of chemotherapy was reduced by a third in patients 60-70 years old and by half in patients over 70 years. The primary objectives were to determine induced complete remission rate and major molecular response of the dasatinib/prednisone oral, chemo-free regimen.
Results
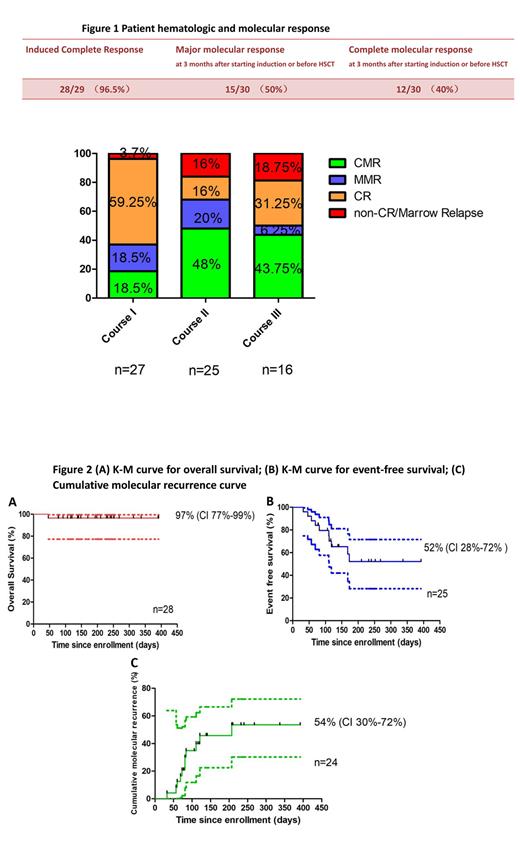
To date, 32 patients from 10 centers enrolled. Median age was 54 years (range: 15-84). The dominant BCR-ABL1 isoform was p190 in 72%, p210 in 18%, rare types in 6.3% and not reported in 3.1%. Patients received an average of 2.5 courses of dasatinib/prednisone regimen (Course I-III). Most patients were tolerable the dose of dasatinib, and two patients were reduced to 50mg daily due to severe pleural effusion. Allo-HSCT was performed in 10 patients, 50% after remission and 50% after marrow relapse.
As shown in Figure 1, the CR rate was 97% with one induction death due to sepsis. The major molecular response (MMR was defined as: 3-log reduction in BCR-ABL1 transcript) was 50% and the complete molecular response (CMR was defined as: the absence of a detectable BCR-ABL1 transcript with 0.01% sensitivity/4.5-log) was 40% either at 3 months after starting induction or before HSCT, respectively. From Course I to Course II, the CMR increased from 18.5% to 48%, but accompanied by an increase in marrow relapse rates, which were 16% and 18.75% at course II and course III, respectively. Marrow relapse occurred in 9 patients, all occurred within 6 months after starting induction. T315I mutation in ABL1 KD was detected in 5 of 7 marrow relapses (72%). With a median follow up of 232 days (range: 77-392 days), 1-year OS and 1-year event-free survival (events were defined as marrow relapse and death) was 97% and 54%, respectively (Figure 2). The cumulative molecular relapse rate (defined as BCR-ABL1:ABL1 ratio rises after reaching the CMR level) was 35% at 3 months, 48% at 6 months, and 54% at 1 year (Figure 2).
Conclusions
The chemotherapy-free, oral combination of dasatinib and prednisone appears promising induction efficacy in de novo Ph+ ALL; the high CR rates and acceptable molecular response rates were similar to results reported by GIMEMA LAL2116 study (Foà R, et al. N Engl J Med. 2020;383:1613-1623). However, two subsequent consolidation courses of the chemo-free regimen led to early molecular relapse with T315I mutations, which urged us to modify the study protocol such as strengthening consolidation therapy or incorporating promising drug blinatumomab.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal