Abstract
Background: Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts caused by a combination of both impaired platelet production and increased peripheral platelet destruction. Current first-line ITP treatments include glucocorticoids and intravenous immunoglobulin (IVIG). However, these drugs have variable and transient efficacy, significant toxicities, and relapse is common upon discontinuation. Subsequent treatment options include the thrombopoietin receptor agonists (TPO-RAs). Avatrombopag (AVA) is an orally administered small molecule TPO-RA. It binds to the human TPO receptor (c-Mpl) at a site that is different from the endogenous TPO binding, stimulating signal transduction and mimicking the biological effects of endogenous TPO. In phase 2 and 3 studies in patients with ITP, AVA was administered to 128 patients for a median duration of 7 months (maximum duration >2 years; Birhiray R, et al. EHA 2020). In a phase 3 trial (NCT01438840), the primary efficacy endpoint of cumulative number of weeks with platelet count ≥50×10 9/L during 6 months of treatment in the absence of rescue therapy was statistically significant favoring AVA over placebo. The most common treatment-emergent adverse events (AEs) in these phase 2 and 3 trials were headache, fatigue, and contusion. AVA has no significant hepatotoxicity and is administered with food without restrictions on meal composition. AVA is approved by FDA and EMA for the treatment of primary chronic ITP in adult patients who are refractory to other treatments (e.g. corticosteroids, IVIG). However, there is a need to provide data to treaters and the ITP community on the real-world usage and effectiveness of AVA (including patients previously treated with TPO-RAs). Described here is the rationale and design of the ADOPT study (NCT04943042), evaluating the use and effectiveness of AVA in adult patients with ITP in routine clinical practice in Europe.
Study design and methods: This is a multicenter, observational, phase 4 study, with the primary objective to describe the real-world effectiveness of AVA treatment over a prospective period of 12 months in adult patients with ITP. Prospective data will be collected at routine clinical visits. In addition, retrospective data will be collected from patients' medical records for up to 12 months prior to AVA treatment start.
Eligible patients must be ≥18 years, have provided informed consent, have an established and well documented ITP diagnosis, and are treated with or initiating treatment with AVA for ITP at enrollment. Decision to initiate treatment shall be made by the treating physician, independently from the decision to include the patient in the study.
Exclusion criteria include enrollment in other clinical interventional study or intake of an investigational medicinal product ≤3 months prior to inclusion, and secondary ITP.
The primary endpoint is cumulative number of weeks with a platelet count ≥30×10 9/L during AVA treatment. Platelet counts during rescue medication use and within 4 weeks after stopping a rescue medication or following splenectomy are considered non-response and thus not included in the primary endpoint cumulative number.
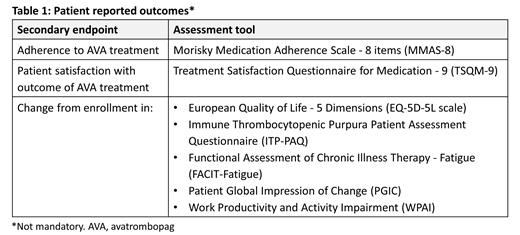
Secondary endpoints supporting the primary objective include cumulative number of weeks with a platelet count ≥50×10 9/L, patients (n [%]) with a platelet count ≥30×10 9/L and ≥50×10 9/L for at least 8 consecutive weeks, patients (n [%]) experiencing WHO bleeding grade ≥2, patients (n [%]) requiring rescue medication, and time from AVA treatment start to platelet count ≥30×10 9/L and ≥50×10 9/L. Additional secondary endpoints are AVA dose and dosing frequency, reason for ITP treatment discontinuation or change from one ITP treatment to another (prior to and during the study), use of concomitant ITP medications, physician satisfaction with outcome of AVA treatment (5-point scale), physician assessment of clinical change of AVA treatment (Clinical Global Impression of Change scale), healthcare resource use, as well as a number of patient reported outcomes (Table 1). Secondary safety endpoints include serious AEs, AEs of special interest (thromboembolic events, significant bleeding) and AEs leading to AVA discontinuation.
Data will be summarized using descriptive statistics.
Study status: The study is planned to start 2021 and aims to enroll 150 patients.
McDonald: Grifols: Research Funding; Bayer, Sobi, Novartis, Amgen, argenx: Honoraria. Matzdorff: Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Current holder of individual stocks in a privately-held company. Mellbring: Sobi: Current Employment. Nazir: Sobi: Current Employment. Lindqvist: Sobi: Current Employment. Santagostino: Sobi: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal