Abstract
Introduction
Lymphoblastic leukemia (ALL) is a neoplasm of immature lymphoid cells of either B- or T-cell lineage. B-ALL is the more common (particularly in childhood), and has a number of described recurrent genetic abnormalities with distinct clinic-pathological associations. T-ALL comprises a larger proportion of adult ALL (18-23%) than childhood cases (7-15%) in high income countries, and is genetically heterogeneous without clear prognostic associations with genetic subtypes. The frequency of T-ALL and the genetic landscape of B-ALL show regional variation. T-ALL is common among African American children (~25%), but seen infrequently in Asia (~7% of childhood cases). In B-ALL, the translocation t(12;21) and hyperdiploidy predominate among children in Europe and the USA, while KMT2A rearrangement and the translocation t(9;22) are relatively more common in Asia. There is a paucity of literature regarding ALL in Africa; the distribution of its subtypes (B vs T), its genetic composition and outcomes are not known. This study aimed to characterize ALL diagnosed in the state-sector hospitals of Johannesburg, South Africa (SA).
Methods
Cases diagnosed with ALL in the flow cytometry laboratory at Charlotte Maxeke Johannesburg Academic Hospital (which provides diagnostic immunophenotyping services to all state-sector hospitals of the southern Gauteng region of SA) between 2016-2019 (42 months) were identified and recorded in a database. Pertinent information was documented from the laboratory information system.
Results
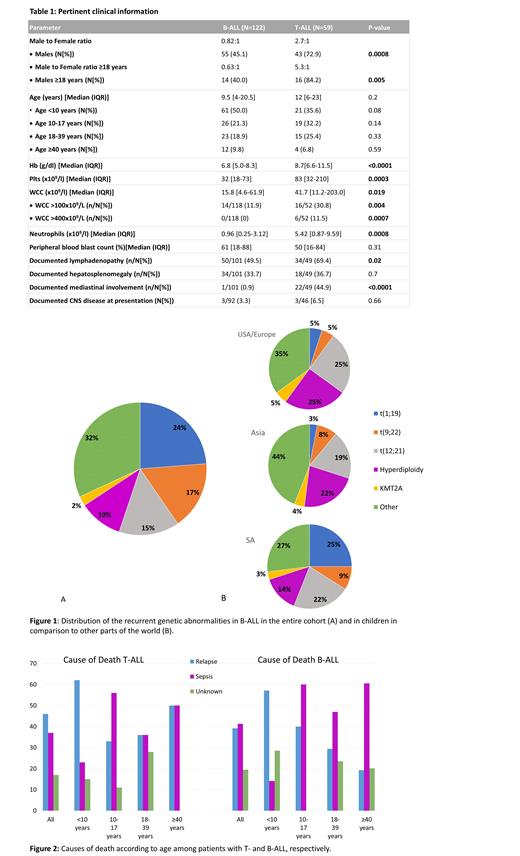
ALL was diagnosed in 181 patients over the time period; pertinent clinical information is reported in Table 1. T-ALL was substantially more common than reported elsewhere, comprising 31.5% and 35.2% of childhood and adult ALL, respectively. Differences were evident in the cytogenetic patterns seen in both B- and T-ALL as compared to other parts of the world. In B-ALL the translocation t(1;19) (which occurs in <10% of cases elsewhere) was the most common recurrent genetic abnormality (23.7%), and the t(9;22) had a relatively high frequency in children <13 years (8.8%) (Figure 1). In T-ALL, karyotypic abnormalities were more common than typical (seen in 80.0% of cases vs 50-70% elsewhere), with derangements of chromosome 6q being the most frequent (19%). The translocation t(10;11) (PICALM-MLLT10) and abnormalities involving the TLX1 (HOX11) and TLX3 (HOX 11L2) genes (which are among the more frequent genetic abnormalities reported internationally) were all uncommon, each occurring in only 2.4% of the cases.
Disease outcomes were substantially poorer compared to those reported in high income countries, where survival rates in childhood T-ALL range from 60-80% and exceed 90% in B-ALL. At a median follow-up time of 36 months, only 68.2% (B-ALL) and 27.8% (T-ALL) of children <10 years were alive, while mortality rates among adults exceeded 80% in both T- (86.7%) and B-ALL (83.3%). Survival in patients with T-ALL did not differ between those with high vs low risk clinical features (age >10 years, white cell count >100 x10 9/L), and was significantly worse as compared to those with B-ALL (p = 0.01). Relapse was the dominant cause of death in children <10 years (more so in those with T-ALL), while death due to chemotherapy-related neutropenic sepsis was more common in older patients (particularly those with B-ALL) (Figure 2). Factors associated with disease relapse in B-ALL included KMT2A rearrangement and measurable residual disease (MRD) after induction chemotherapy (as defined by non-quantitative, non-allele specific PCR of IgH/T-cell receptor gene rearrangement status and 4 color flow cytometry (both with sensitivities >0.1%)). Notably, the high risk of relapse associated with MRD was not seen in patients with t(9;22), likely due to the use of targeted molecular therapy in these cases. No significant predictors of survival were identified in T-ALL, but the presence of MRD post-induction was associated with early death due to relapse (<12 months).
Conclusion
ALL in SA shows distinct differences in the cytogenetic landscape, disease patterns and outcomes. The cause of the poor survival rates likely includes differences in tumour/host biology, late presentation, restricted access to haemopoietic stem cell transplantation in the SA state-sector, and suboptimal neutropenic support. Although rudimentary, available MRD testing is a valuable risk predictor in both B- and T-ALL.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal