Abstract
Background
High-dose melphalan (HDM) at 200 mg/m2 is a myeloablative consolidation treatment prior to autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM) and is administered in 1-day or divided over 2-days. Although the 1-day regimen (lower AUC) has been shown to result in significantly less gastro-intestinal toxicity, it is not completely clear whether this administration strategy has any deleterious effects on efficacy, compared to the 2-day regimen. In this retrospective cohort study, we aimed to evaluate the effects of 1- or 2-day dosing of HDM on disease remission, progression-free survival (PFS) and overall survival (OS) in patients with MM.
Methods
Data from two academic centers in Amsterdam that have recently merged were used for the analysis, with one of the centers using the 1-day regimen and the other the 2-days regimen. A total of 265 patients with MM divided over the 1-day group (n=174) and 2-day group (n=91) treated between July 2017 and February 2020, were included in the study. The primary endpoint was the proportion of patients with at least very good partial remission (≥VGPR) at day ±90 post-ASCT. Secondary endpoints were overall survival (OS), progression-free survival (PFS), duration of hospitalization post-ASCT, engraftment period of neutrophils and platelets, and complications (other than mucositis) during hospitalization.
Results
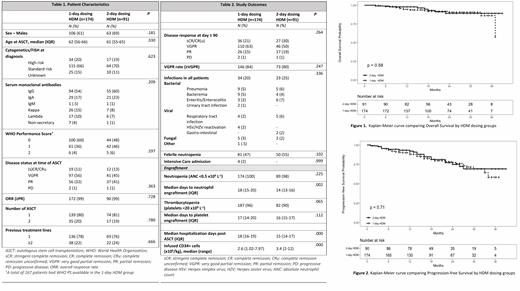
Patient characteristics are summarized in Table 1. Remission status of ≥VGPR was comparable between the 1-day and 2-day groups (84% vs. 80% respectively). After a median follow-up of 21 months, OS (92% vs. 91%) and PFS (80% vs. 81%) were comparable in the 1-day and 2-day group respectively. There were no differences in the incidence of hematologic adverse events between the 1-day and 2-day groups (neutropenia; 98% vs. 100%, thrombocytopenia; 90% vs. 96% respectively). Median time to neutrophil engraftment (ANC >0.5 x10 9 L -1) was significantly shorter in the 2-day group than in the 1-day group (14 days vs. 18 days, p = 0.002). Median time to platelet engraftment (platelets >20 x10 9 L -1) was comparable between the groups. Lower CD34+ cell counts were administered in the 1-day group compared to the 2-day group (2.6 vs. 3.4 x10 6/kg, p <0.0001). A significant negative correlation between the reinfused CD34+ cell counts and time to neutrophil engraftment was found (R= - 0.244). Table 2 shows the number of hospitalization days after ASCT. The median number was 18 days in the 1-day group and 15 days in the 2-day group (OR 1.22, 95% C.I. (1.10-1.35), p <0.0001). Incidences of infectious complications, febrile neutropenia and intensive care unit (ICU) admissions were not different between the groups.
Conclusion
The use of 1-day HDM as consolidation treatment in MM patients resulted in equal disease response, progression-free survival and overall survival as compared to 2-day HDM. Based on the results of this study showing comparable efficacy and earlier findings of reduced toxicity with the 1-day HDM administration, we recommend the 1-day protocol for HDM. Interestingly, our results also confirmed that patients might benefit from higher counts of reinfused CD34+/enucleated cells.
Wondergem: Novartis: Honoraria. de Leeuw: Takeda: Membership on an entity's Board of Directors or advisory committees. Biemond: Sanquin: Research Funding; Celgene: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Global Blood Therapeutics: Honoraria, Research Funding, Speakers Bureau. van de Donk: Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding. Zweegman: Sanofi: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nur: Roche: Speakers Bureau; Celgene: Speakers Bureau; Novartis: Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal