Abstract
INTRODUCTION
The use of post-transplantation cyclophosphamide (PTCy) for graft-versus-host disease (GvHD) prophylaxis has decreased the rates of this complication, resulting on an improvement of transplant-related toxicity and survival. Secondary to its efficacy, the use of PTCy has been almost universally integrated for allogeneic hematopoietic cell transplantation (alloHCT), independently of the selected donor source.
Clinical decisions in alloHCT are supported by the use of prognostic scores for outcome prediction. However, capability of prediction by diverse scores can vary depending on their features and on the composition of the study cohort. Additionally, the continuous innovation on alloHCT techniques and practices leads to an ongoing need to update risk indices aimed at improving risk stratification of patients undergoing alloHCT. This study explores the predictive capacity of different prognostic scores routinely used in alloHCT, in a contemporaneous cohort of adults undergoing peripheral blood (PB) alloHCT using PTCy-based GvHD prophylaxis.
METHODS
Between 2014 and 2020, 230 consecutive adults with hematological malignancies underwent PB-alloHCT with PTCy-based GvHD prophylaxis at our Institution. Data related to Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), Karnosfky Performance Status (KPS), Disease Risk Index (DRI), European Bone Marrow Transplantation (EBMT) score, and Endothelial Activation and Stress Index (EASIX) were collected retrospectively. Complete information was available for 216 patients. Overall survival (OS) was considered the main outcome variable. Patients were grouped into two risk groups based on the optimal cut-off value for each score. In the case of EASIX, 1.578 was the most discriminating cut-off for OS. The score discrimination for OS was measured independently for each index using the receiver operating characteristic curve (AUC) calculated using receiver operating characteristic (ROC) curves, and determined at different time-points after alloHCT.
RESULTS
Of the 216 patients included, the median age was 52 years (range: 18-70), acute myeloid leukemia (36.1%) was the most prevalent baseline diagnosis, 42.1% of adults underwent reduced-intensity conditioning alloHCT, 69.4% received grafts from unrelated donors, and 23.0% from haploidentical donors. With a median follow-up of 22.6 months, 24.1% patients relapsed, and 2-y OS and non-relapse mortality were 67.3% and 19.9%.
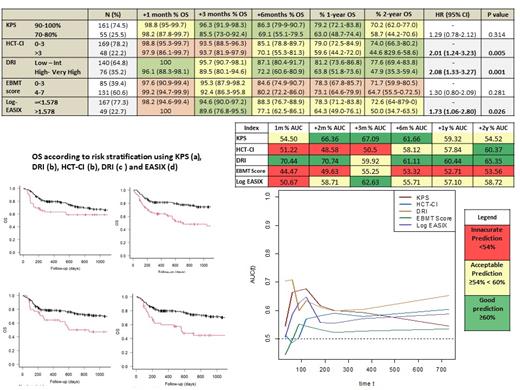
DRI, HCT-CI, KPS, and EASIX successfully grouped patients into higher and lower risk strata, supporting their use for risk classification. HCT-CI [(score>3 (vs 0-3): HR 2.02, p<0.01], DRI [High - Very High risk (vs Low - Int): HR 2.08, p<0.01], and EASIX [>1.578 (vs ≤ 1.578): HR 1.73, p<0.02], maintained an optimal discrimination capacity during the entire post-transplant follow-up (median AUC ranges > 55%).
DRI was the most accurate prognostic index during the entire post-transplant period (median AUC ranges > 60%). KPS score was found to be a useful predictor of mortality up to the first year after alloHCT and with the highest prognostic accuracy at 3 months (AUC 67.09%). HCT-CI score was found to present a better discrimination capacity once elapsed 6 months after alloHCT and with a peak of prediction capacity at 2 years (AUC 60.3%). EASIX, when measured at the pre-transplant evaluation, demonstrated to have acceptable predictive ability during the entire post-transplant period (median AUC > 55%), and with a peak of prediction at 3 months (AUC 62.6%). The EBMT score had the lowest predictive capacity in our analysis (Figure 1).
CONCLUSION:
This study validates, for the first time, the risk stratification capacity for OS of DRI, HCT-CI, KPS, and EASIX in PB-alloHCT with PCTy-based prophylaxis. Interestingly, the prediction accuracy of the prognostic scores differed depending on the time-period. This result can be taken into consideration to enhance the applicability of these scores and refine the clinical decisions taken based on the information provided from their use in routine clinical practice.
Lozano: Terumo BCT: Honoraria, Research Funding; Macopharma: Research Funding; Grifols: Honoraria. Rosinol: Janssen, Celgene, Amgen and Takeda: Honoraria. Esteve: Novartis: Consultancy, Research Funding; Astellas: Consultancy; Jazz: Consultancy; Pfizer: Consultancy; Novartis: Research Funding; Abbvie: Consultancy; Bristol Myers Squibb/Celgene: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal