Abstract
Keywords
CD19, fourth-generation CAR-T cell therapy, diffuse large B cell lymphoma, anti-PD-1 antibody
Background
With the advance in CD19 CAR-T therapy, there have been improvements in the treatment of refractory/relapsed diffuse large B cell lymphoma (R/R DLBCL). Still, many genetic modifications on this CAR-T product or combination agents are being explored to improve the immunosuppressive tumor microenvironment, CAR-T cell exhaustion or overcome other limitations. Using a series of in vitro studies, we demonstrated that IL-7 and CCL19 prominently promoted the cytotoxicity and the expansion of CAR-T cells. On the other hand, the existence of different immunosuppressive pathways such as PD-1/PD-L1 pathway can restrict the full potential of CAR-T therapy. Thus, it is reasonable to postulate that CD19-specific CAR-T cells that express both IL-7 and CCL19 (CD19-7×19 CAR-T cells) in combination with anti-PD-1 antibody may constitute a potential option for R/R DLBCL. Here, we present the preliminary results from a groundbreaking clinical trial of CD19-7×19 CAR-T cells plus anti-PD-1 antibody which evaluates the safety and efficacy of this new strategy.
Methods
This phase Ib, single-arm, open-label, single-center trial enrolled 8 patients (18-75 years) with R/R DLBCL. Lymphoma biopsies were immunostained for various target antigens including CD19. PD-L1 expression was confirmed by immunohistochemistry using an anti-PD-L1 mAb. Autologous T cells were apheresis collected and transduced with a safety-engineered lentiviral CAR with the following intracellular signaling domains: CD8/4-1BB/CD3ζ/IL-7/T2A/CCL19 (4SCAR). CD19-7×19 CAR-T cells were administrated at dose of 1 to 3×10 6 CAR-T cells/kg following lymphodepleting chemotherapies using fludarabine (30 mg/m 2) and cyclophosphamide (500 mg/m 2). At 30th day after modified T-cells infusion, patients received 6 cycles of anti-PD-1 antibody Tislelizumab (200mg) for every 3 weeks. The primary endpoints were safety and objective response rate (ORR). The key second endpoints included 2 years PFS, 2 years OS, DOR, blood CAR copies, and cytokine profiles. Adverse events (AEs) were defined according to CTCAE 5.0. Efficacy of the treatment was assessed by F-FDG PET/CT at 3 months after CAR-T infusion.
Results
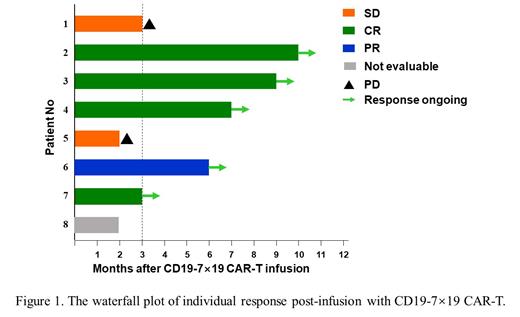
At the data cutoff, 8 patients had received CD19-7×19 CAR-T cells, including 7 males and 1 female. The median age was 45.5 years old (range, 38-65). The performance status of the 8 patients was Eastern Co-operative Oncology Group score 0 to 3 at the time of infusion. Patient characteristics include 4 with stage IV disease (50%), 1 after autologous stem cell transplantation (12.5%), 2 with bone marrow involvement (25%), and none of them received prior PD-1 antibody treatment. The average transduction efficiency of CAR was 30.625%. Among the 8 pts, 3 received infusion dose of 1 × 10 6/kg, 3 received the dose of 2 × 10 6/kg and 2 received the dose of 3 × 10 6/kg. 2 patients (25%) developed greater than grade 2 cytokine release syndrome and 2 (25%) developed neurotoxicity (grade 3). These adverse effects resolved quickly after intervention. Total 7 patients were evaluated at three months follow-up time, resulting in 4 complete response (CR), 1 partial response (PR), and 2 disease progression (PD). The overall response rate was 5/7 and CR rate was 4/7. Moreover, another R/R DLBCL patient with stage Ⅳ disease and a bulky mass in the liver (>12 cm) receiving compassionate CAR-T therapy, who wasn't enrolled because of hepatitis B virus infection, achieved and still remained in continuous CR over 6 months.
Conclusion
These results showed the feasibility, controllable toxicities, and marked response rate with this potential approach for R/R DLBCL. However, it remains unclear whether long term remission rate can be achieved. Long term follow-up and additionally enrolled patients would be necessary.
Disclosures
The authors declare that they have no competing interests.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal