Abstract
Background: Bridging therapy (BT) was not allowed in the ZUMA-1 pivotal trial for axicabtagene ciloleucel (axi-cel) chimeric antigen receptor T-cell therapy (CAR-T) . Since then, several real-world studies have shown the use of bridging therapy to be associated with worse overall survival, duration of response, and complete remission rates. In addition, patients requiring BT during CAR-T manufacturing have a more aggressive and higher tumor burden of disease, also factors associated with poor outcomes. Therefore, factors that can predict outcomes in this high-risk patient cohort are required. We herein examine the impact of response to BT on CAR-T outcomes in large B-cell lymphoma (LBCL).
Methods: A retrospective review of patients who received axi-cel for NHL from June 2016 - July 2020 at Mayo Clinic, Rochester, was performed. BT was defined as any lymphoma-directed therapy given between leukapheresis and CAR-T infusion. Patients received BT if there were concerns for symptomatic progression of disease during CAR-T manufacturing, reducing the likelihood of eligibility to receive CAR-T. The decision and choice of BT were at the discretion of the treating physician. Response to all lymphoma-directed therapy was evaluated using the 2014 Lugano criteria. Response to BT included patients with a partial response (PR) or stable disease (SD) on PET-CT before initiating lymphodepletion chemotherapy. Event-free survival (EFS) was defined as the time from axi-cel infusion to progression, next treatment, or death. Overall survival (OS) was defined as the time from axi-cel infusion to death. Survival curves were calculated using Kaplan-Meier estimates and were compared between subgroups using the log-rank test. Cox regression was used for univariate and multivariate analysis (MVA).
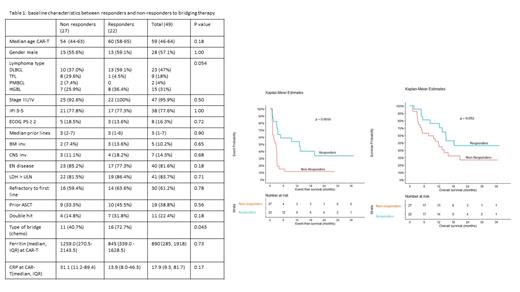
Results: A total of 73 patients underwent car T therapy during this period. Of these, 67% (49/73) received BT therapy. Table 1 shows baseline characteristics of the total BT cohort (n = 49). The median age at CAR-T infusion was 59 years (IQR 46-64); 57% were males and comprised of 47% (23/49) DLBCL followed by 31% (15/49) high-grade B-cell lymphoma types. Based on the Lugano criteria on PET-CT, 22/49 (45%) patients responded to BT. The baseline characteristics were comparable between the responders and non-responders to BT except for a higher proportion (73%) of patients receiving systemic chemotherapy as BT in the responders (Table 1). At a median follow-up of 24 months, 75% had either progressed, died, or started the next treatment (event), and 59% (29/49) had died. The median EFS was significantly longer in the responders as compared to the non-responders to BT, figure 1 (13.04 months (95%CI, 3.54-not reached [NR]) vs. 2.56 months (95%CI, 1.18-3.02), p = 0.002). The OS also trended in favor of the responders (median OS 18.4 months (95% CI, 13.44-NR) vs. 11.84 months (95% CI, 5.05-NR), p = 0.092). The responder group also had a higher 6-month CR rate of 50% than 11.1% in the non-responder group (p = 0.004). There were no differences in any grade or grade ≥ 3 cytokine release syndrome and neurotoxicity rates in the two groups. On univariate analysis within the bridging group (n = 49), type of bridge (non-chemo) and response to bridge (PR+SD) were associated with a better EFS. In the MVA, only response to BT maintained significance for EFS (HR 0.34, p = 0.025).
Conclusions: Having some control of lymphoma after BT was associated with better EFS and 6-month CR rate. Future studies need to prospectively evaluate the type and response to BT as a prognostic factor for improving outcomes in patients receiving CAR-T.
Wang: InnoCare: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Genentech: Research Funding; MorphoSys: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Paludo: Karyopharm: Research Funding. Bennani: Kymera: Other: Advisory Board; Vividion: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Purdue Pharma: Other: Advisory Board; Verastem: Other: Advisory Board. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Lin: Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy, Research Funding; Bluebird Bio: Consultancy, Research Funding; Juno: Consultancy; Legend: Consultancy; Sorrento: Consultancy; Gamida Cell: Consultancy; Vineti: Consultancy; Merck: Research Funding; Takeda: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal