Abstract
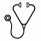
Background: CD34 expression remains the most common immunophenotypic cell surface marker defining human hematopoietic stem and progenitor cells (HSPCs). Recently, use of multicolor fluorescence-activated cell sorting (mFACS) and single-cell RNA sequencing (scRNA seq) has illustrated the heterogenous nature of CD34+ HSPCs, with immunophenotypically and transcriptionally distinct subsets ranging from primitive hematopoietic stem cells (HSCs) capable of long-term multilineage potential to differentiated, lineage-committed progenitors. Meanwhile, the addition of CXCR4 inhibitors (CXCR4i) to G-CSF (G) has increased mobilization of CD34+ HSPCs for stem cell transplantation (SCT). Yet, the effect of CXCR4i +/- G on mobilization of specific immunophenotypic and transcriptional CD34+ HSPC subsets is not well-characterized. Motixafortide (M) is a novel cyclic peptide CXCR4i with a low receptor off rate and extended in vivo action vs plerixafor (Px). M was recently evaluated in the phase 3, double blind, placebo controlled GENESIS Trial as an HSPC mobilizer prior to autologous SCT (ASCT) in multiple myeloma (MM).
Methods: GENESIS Trial patients were prospectively randomized (2:1) to receive either M+G or placebo (P)+G for HSPC mobilization. Demographically similar patients undergoing mobilization with Px+G prior to ASCT for MM were prospectively enrolled on a parallel tissue banking protocol. All patients received G (10 mcg/kg) on days 1-5 (and 6-8 if needed). Patients also received either M (1.25 mg/kg) or P on day 4 (and 6 if needed); or Px (0.24 mg/kg) on day 4 (and 5-7 if needed). Apheresis began day 5 (and 6-8 if needed). HSPCs were purified from apheresis product on day 5 via CD34+ immunomagnetic selection. CD34+ HSPC subset profiling was performed via mFACS and scRNA seq. CXCR4 expression and receptor occupancy was evaluated by antibody binding capacity of 12G5 and 1D9 clones.
Results: Demographics were similar between the M+G (n=24), P+G (n=13) and Px+G (n=14) cohorts. By mFACS, M+G mobilized a 5.5 fold higher absolute number (#) of HSCs, multipotent progenitors (MPP) and common myeloid progenitors (CMP) vs P+G (p<0.0001); and a 2.6 fold higher # of these subsets vs Px+G (p=0.0327) (Figure 1A). M+G mobilized a 10.5 fold higher absolute # of primitive HSCs vs P+G (p<0.0001) and a 1.1 fold higher # vs Px+G (p=0.7876) (Figure 1B). M+G and Px+G mobilized a 7-8 fold higher # of plasmacytoid dendritic cell precursors vs P+G (p-values >0.05). 1D9 binding to CXCR4 on CD34+ HSPCs was similar between all 3 arms (p-values 0.45-0.75). 12G5 binding (which competes with CXCR4i's for binding to CXCR4) was significantly lower with M+G (MFI:11) vs P+G (MFI:74; p<0.0001) and Px+G (MFI:271; p<0.0001).
By scRNA seq, UMAP clustering identified 3 transcriptionally similar HSC sub-clusters (HSC-I, -II and -III) mobilized by all 3 regimens; and 1 distinct HSC-PL cluster mobilized by P+G expressing heat shock protein genes (HSPA1 A/B) (Figure 1C). Differentially expressed genes (DEGs) of HSCs I-III included CD52, FTH1, HLA-E, KLF2 and LMNA. AVP was unique to HSC-I while EGR1, JUN and ZFP36 were unique to HSC-III. MPPs clustered into 3 sub-clusters (MPP-I, -II and -III). MPP-I clustered closely to HSC-II/III with low expression of genes of differentiation. MPP-II expressed DEGs (PLEK) on a continuum toward megakaryocyte-erythroid progenitors (MEP-I/II), which expressed DEGs of erythroid differentiation (HBD/HBB). MPP-I and -II contained cells from all 3 regimens. However, MPP-III was specific to M+G with DEGs (MT-ATP8, HIST1H1) associated with monocyte, lymphocyte and NK cell differentiation.
Conclusions: Extended CXCR4i with M+G mobilized significantly higher #s of combined CD34+ HSCs, MPPs and CMPs vs Px+G and P+G (p-values <0.05). Additionally, M+G mobilized a 10.5 fold higher # of immunophenotypically primitive CD34+ HSCs capable of broad multilineage hematopoietic reconstitution vs P+G (p<0.0001) and similar #s vs Px+G. CXCR4 expression on CD34+ HSPCs by 1D9 binding was similar across all arms. Whereas, 12G5 binding was much lower with M+G vs P+G and Px+G, consistent with extended CXCR4 receptor occupancy by M. All regimens mobilized transcriptionally similar HSC-I-III subsets. However, lack of CXCR4i (M or Px) resulted in mobilization of more-differentiated HSCs (HSC-PL subset). Whereas extended CXCR4i with M+G (but not Px+G) mobilized a unique MPP-III subset with DEGs related to leukocyte differentiation.
Crees: BioLineRx Ltd.: Research Funding. Retting: BioLineRx Ltd.: Research Funding. Jayasinghe: WUGEN: Consultancy; MMRF: Consultancy. Vainstein: BioLineRx LTD: Current Employment. Sorani: BioLineRx LTD: Current Employment. Ickowicz: BioLineRx Ltd.: Current Employment. Shemesh-Darvish: BioLineRx LTD: Current Employment. Kadosh: BioLineRx Ltd.: Current Employment.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal