Abstract
Acute lymphoblastic leukaemia (ALL) is the most common pediatric haematological malignancy with a prevalence of around 85% cases of B cell origin. ETV6-RUNX1 is the most common genetic aberration in childhood B-ALL with a prevalence of around 25% in Western countries and a lower prevalence in Asian countries. ETV6-RUNX1 presence portends a good prognosis in B-ALL. ETV6-RUNX1 translocation has been observed in 1-5% of healthy newborns, indicating that it is not sufficient for leukemogenesis, and the need to accumulate secondary mutations for leukemogenesis.
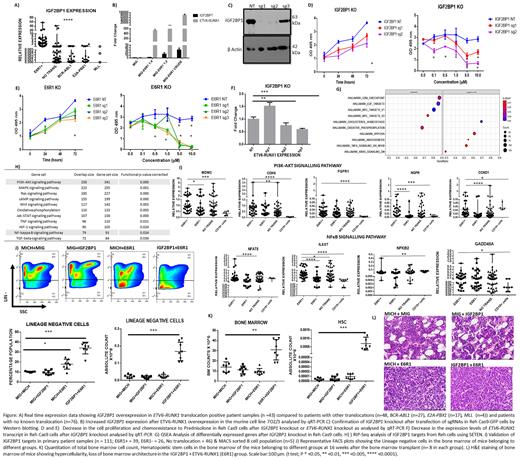
We have previously demonstrated the specific overexpression of Insulin like Growth Factor 2 Binding Protein 1 (IGF2BP1), an oncofetal RNA binding protein, in ETV6-RUNX1 positive B-ALL. Overexpression of the ETV6-RUNX1 fusion protein in 7OZ/3, a murine B-ALL cell line increased endogenous Igf2bp1 levels in a dose dependent manner.
Conversely, knockout of IGF2BP1 in Reh-Cas9-GFP expressing B-ALL cell line, a modified version of Reh (cell line positive for ETV6-RUNX1 translocation), using 2 different guide RNAs led to a decrease in the ETV6-RUNX1 transcript expression in the IGF2BP1 KO Reh-Cas9 cells. This was accompanied by a decrease in cell proliferation and increased chemosensitivity to prednisolone.
A similar phenotype was observed in Reh-Cas9 cells after knockout of ETV6-RUNX1 fusion transcript hinting towards a synergism between the two proteins in tumour cell survival and chemosensitivity. Interestingly, there appears to be a positive correlation between prednisolone resistance and ETV6-RUNX1 positivity in our patient cohort too, thus supporting the in vitro finding. Previous studies have shown the binding of IGF2BP1 to the ETV6-RUNX1 fusion transcript which was also confirmed by us.
To study the in vivo synergistic effect between ETV6-RUNX1 fusion protein and IGF2BP1, we used a murine bone marrow transplant model. The individual genes were overexpressed alone (IGF2BP1/ETV6-RUNX1) or together (IGF2BP1+ETV6-RUNX1) in separate groups of lethally irradiated mice (n=8 per group). In the group where both the genes were overexpressed together, there was clonal expansion and hypercellularity in the bone marrow. This was accompanied by an increase in the number of lineage negative cells (Lin-), progenitors including HSCs, LMPPs and c-kit+ cells which also showed high Ki67 positivity. The marrow hypercellularity led to extramedullary haematopoiesis which was seen as significant splenomegaly.
In addition, analysis of the endogenous levels of Igf2bp1 in the bone marrow of the primary murine samples belonging to the ETV6-RUNX1 fusion protein overexpression group resulted in an increase in its levels, thus confirming our in vitro finding suggesting its induction by ETV6-RUNX1 fusion protein.
RNA-Seq of IGF2BP1 KO cells identified enrichment of targets of the TNF alpha/NFκB signalling and K-Ras pathways hinting towards their dependency on IGF2BP1. The direct targets of IGF2BP1 were identified by RIP-Seq (RNA-Immunoprecipitation-Seq) analysis in Reh cell line. Numerous genes from the oncogenic signalling pathways including the TNF alpha/ NFκB signalling pathway were identified as IGF2BP1 targets. We validated some overexpressed genes from the TNF alpha/NFκB signalling and PI3K pathway in primary patient samples. We found significant overexpression of some of the genes (IL6ST, NFAT5, CDK6, MDM2, CCND1, NGFR) belonging to these pathways in the ETV6-RUNX1 positive group (n=39) compared to the negative cohort (n=111) as well as MACS sorted CD19+ B-cells (n=5). The decrease in the ETV6-RUNX1 transcript levels after downregulation of IGF2BP1 and overlap between the oncogenic pathways clearly shows an interdependency between the two genes.
Overall, our results suggest a potential feedback mechanism between ETV6-RUNX1 fusion protein and IGF2BP1 in the pathogenesis of leukemia development and IGF2BP1 can be utilised as an ideal therapeutic target for this particular subtype.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal