Abstract
Close surveillance of measurable residual disease (MRD) following completion of therapy for acute myeloid leukemia (AML) enables early detection of relapse in time for pre-emptive treatment. Today this is available only for the fraction of patients with recurrent targets quantifiable with standardized RT-qPCR. Patient-tailored MRD monitoring based on leukemia-specific mutations may be an attractive option in patients lacking such aberrations. In this population-based study, we used whole exome sequencing (WES) to identify leukemia-specific mutations suitable for MRD monitoring, and targeted deep sequencing (deep seq) and droplet digital PCR (ddPCR) for quantitative monitoring in children with AML. We investigated the clinical applicability of patient-tailored deep seq and ddPCR for early detection of AML relapse in peripheral blood (PB).
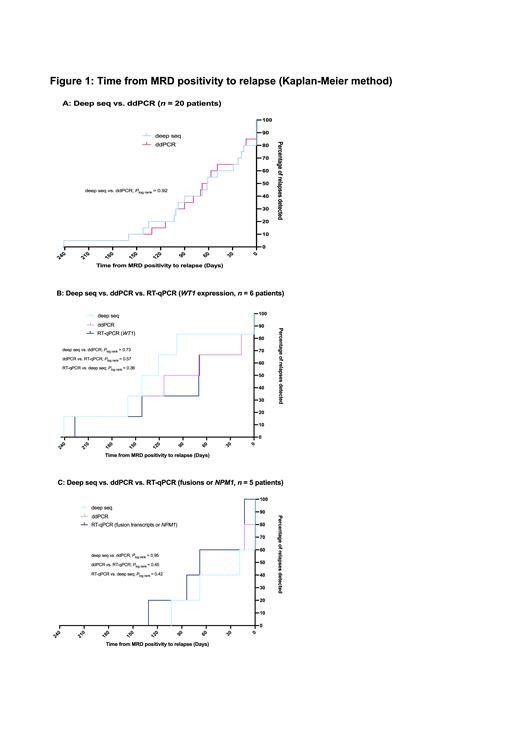
All children (0-18 years) with de novo AML diagnosed between March 2013 and December 2018 who achieved complete remission after treatment according to the NOPHO-DBH AML 2012 protocol (EudraCT number: 2012-002934-35) in Sweden, Denmark, the Netherlands, Norway and Finland were eligible for the study (n = 179). In the study, patients had PB samples drawn at monthly intervals and biobanked for retrospective analyses of leukemia-specific mutations. In patients with a marker for RT-qPCR, samples were also analyzed with RT-qPCR with results reported back to the clinic. Included patients (n = 134) had PB samples drawn starting one month after last consolidation course or date of allogeneic stem cell transplantation and until relapse or end of follow-up (12-18 months following therapy completion), (median 11 samples/patient, range 1-28). Out of the 34 patients who experienced relapse during the study period, sufficient biological material for WES at diagnosis was available from 26 patients (21 hematologic and 5 molecular relapses as determined by RT-qPCR in patients with a suitable marker). For these 26 patients, the median time from end of therapy to relapse (hematologic or molecular) was 6.7 months (range 3.1-23.7). In 24/26 patients, leukemia-specific mutations could be identified at diagnosis. For 22/24 patients, leukemia-specific mutations were verified to be present at relapse (hematologic or molecular) either by WES or deep seq. In the remaining two patients with molecular relapse, leukemia-specific mutations were not verified at molecular relapse. In the 22 patients where leukemia-specific mutations could be detected, 55 mutations (median 3/patient, range 1-4) were quantified with deep seq (limit of detection [LoD] 0.02% variant allele frequency [VAF]) in 111 PB samples (263 analyses) with a sampling interval of 0.9 months (range 0.2-2.6). Parallel ddPCR analyses of 43 mutations from 20 of these patients were performed in 100 PB samples (206 analyses) with a median LoD of 0.04% VAF (range 0.003-0.1%). Deep seq and ddPCR were concordant in 187/206 (91%) of analyses and 84/100 (84%) of analyzed samples. Median VAF in the 113 paired analyses with MRD level above LoD was 0.34% (range 0.02-47.23) with deep seq and 0.38% (range 0.02-45.97) with ddPCR (P = 0.7). Obtained VAFs correlated strongly between the two methods (Spearman's ρ 0.91, P < 0.0001). Nineteen of the analyzed patients experienced hematologic relapse and in 17 of these, at least one mutation was detected in blood before relapse. The median lead time from MRD positivity to hematologic relapse was 3.2 months (range 0.6-7.9) when analyzed with deep seq (n = 17) and 2.6 months (range 0.5-7.9) when analyzed with ddPCR (n = 15). Lead times in patients analyzed with both deep seq and ddPCR showed comparable Kaplan-Meier curves (Fig. 1A). Additional comparison of the lead times achieved with deep seq/ddPCR and RT-qPCR of WT1 expression (n = 6) and recurrent genetic aberrations (n = 5) showed similar Kaplan-Meier curves (Fig. 1B-C). As assessed by deep seq in patients who were not pre-emptively treated, relapse kinetics varied greatly between patients; median VAF doubling time was 12 days with a range of 4-43 (n = 17).
In conclusion, highly sensitive quantification of leukemia-specific mutations in a patient-tailored manner using deep seq or ddPCR enables post-treatment monitoring for the majority of children with AML. Frequent assessment in blood may allow for timely identification of pending relapses to initiate pre-emptive treatment strategies.
Abrahamsson: wedish Children´s Cancer Foundation. Research grants and 50% senior research position for clinical research on pediatric leukemia: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal