Abstract
Introduction:
Hepcidin, the master regulator of iron economy, is decreased during pregnancy to facilitate adequate iron transfer across the placenta. Conversely, iron need increases substantially during pregnancy often leading to iron deficiency and subsequently anemia. The PREG-01 Study compared the efficacy and safety of intravenous (IV) ferric derisomaltose (FDI) vs. oral iron in treating persistent iron deficiency in pregnant women. The study found FDI to be efficacious and well-tolerated in pregnancy and the proportion of non-anaemic patients throughout the course of the study was significantly lower in the FDI group. In this analysis, we investigated the effect of baseline hepcidin on the response to IV and oral iron therapy.
Methods:
PREG-01 was a single-centre, open-label, randomized controlled trial. Women 14-21 weeks pregnant with persistent iron deficiency (ferritin<30 µg/L despite oral iron treatment) received a single intravenous 1000 mg dose of FDI (n=100) or 100 mg elemental oral iron daily combined with ascorbic acid (n=101). Hemoglobin (Hb), ferritin and transferrin saturation (TSAT%) levels were captured at baseline and monitored throughout the study. The effect of baseline hepcidin on achieving non-anemic status (Hb ≥ 11 g/dL) at all study visits and the effect on change in Hb, ferritin and TSAT% were investigated by estimating odds ratios from a logistic regression model with treatment as factor and interaction between treatment and baseline hepcidin. The odds ratio estimate is for an increment in baseline hepcidin of 1 ng/mL.
Results:
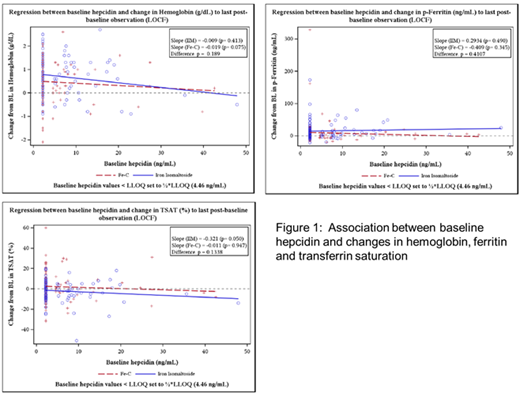
Mean [standard deviation (SD)] baseline Hb was 11.97 (0.93) g/dL in the FDI group and 11.75 (0.91) g/dL in the oral iron group. Baseline hepcidin was 6.42 ng/mL in the FDI and 5.32 ng/mL in the oral iron group. Baseline hepcidin was not associated with the ability to achieve non-anaemic status either in the FDI (OR 0.98; 95% CI: 0.87-1.09) or the oral iron group (OR 0.96; 95% CI: 0.88-1.05). No statistically significant associations were found between baseline hepcidin and change in Hb, ferritin or TSAT% throughout the study (Figure 1).
Conclusions:
In a population of pregnant women with iron deficiency, but otherwise healthy, baseline hepcidin was overall low. Although there was a trend for an association between baseline hepcidin and Hb response to oral iron only, baseline hepcidin did not predict the response to iron therapy.
No relevant conflicts of interest to declare.
Ferric Derisomaltose is an IV iron preparation indicated for the treatment of iron deficiency anemia in the US.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal