Abstract
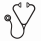
Background: Over the past year, COVID-19 was declared a global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulting in over 100 million cases and >3 million deaths worldwide according to the World Health Organization (WHO). Morbidity and mortality have been highest in adults, particularly in those with underlying conditions including hypertension, diabetes, and obesity. Children, thus far, have largely remained either asymptomatic or presented with mild symptoms. However, sickle cell disease (SCD) was classified as a risk factor for severe COVID-19 disease in both adults and pediatric patients.
Objective: To describe the one-year experience of the clinical course, management, and treatment of COVID-19 in children, and young adults with SCD at Children's National Hospital (CNH).
Methods: This was a single-center, observational cohort study of 55 children (age <18 years) and young adult (age ≥ 18 years) patients with SCD and PCR confirmed SARS-CoV-2 infection at CNH between March 31, 2020, and February 12, 2021 (Figure 1A).
Results: Sixty-nine percent were children (N=38) and 31% were young adults (N=17). The mean age was 11.6 years with 51% females (N=28) and 49% males (N=27). Seventy-five percent of cases were Hgb SS, 15% Hgb SC, and 11% Hgb SBeta Thalassemia Zero (Table 1). Fever (45%) was the most common presenting symptom; only 9% had a loss of taste or smell (Figure 1B). Twenty-two percent were asymptomatic at presentation. Among the 40 patients who presented to the emergency department (ED) or were hospitalized, 50% (N=20) presented with vaso-occlusive pain crisis (VOC), 42% with Acute Chest Syndrome (ACS), 2% with splenic sequestration, and 2% with Venous Thromboembolism. Only 3% were admitted to the ICU (N=2 young adults, N=1 children); none of whom were on hydroxyurea. There were no differences in hospitalization rates between Hgb SS and Hgb SC patients (Table 1). Lower oxygen saturation (02 Sat)(02 Sat <95%: 62% vs 16% p=0.004), fevers (69% vs 16%, p=0.001), VOC pain crisis (50% vs 24%, p=0.047), and ACS (65% vs 3%, p<0.001) were more common in hospitalized patients vs. non-hospitalized patients.
Patients with ACS experienced a longer length of stay (6 days vs. 3 days p=0.008), lower oxygen saturation (02Sat<95%: 81% vs 13% p<0.001), higher white blood cell count (13.3 vs 9.0 K/mcL, p=0.009), lower hemoglobin nadir (6.8 vs. 9.6 gm/dL, p= 0.049), and elevated D-dimers (4.1 vs. 0.8 ug/mL, p=0.002) compared to those without ACS. The types of treatment received by patients requiring hospitalization or ED visits included ceftriaxone (N=28, 70%), azithromycin (N=15, 37.5%), remdesivir (N=6,15%) convalescent plasma(N=1,2.5%). Blood transfusion was required in 29% of the 55 SCD COVID-19 cases (N=16) and 76% (N=13) of the ACS patients. Twenty-six percent (N=17) of hospitalized patients were anticoagulated with either enoxaparin or rivaroxaban according to CNH's COVID-19 anticoagulation treatment protocol. There were similar rates of healthcare utilization, SCD modifying therapies, acute COVID-19 clinical presentation, respiratory support, and laboratory findings (hematologic, inflammatory) between the children and young adults. However, young adults were more likely to be on crizanlizumab treatment (18% vs 0%, p=0.026) and have an elevated D-Dimer (4.0 vs 1.0, p=0.012) on laboratory evaluation.
Conclusion: Our case series reveals that the demographics and clinical presentation between our SCD children and young adult patients with COVID-19 were similar overall. While the morbidity was high, there was no mortality. Those that were hospitalized had lower oxygen saturation levels, higher incidences of fever, and higher morbidity presenting with VOC and ACS. Patients with ACS and/or an oxygen requirement had significantly higher WBC count, lower nadir hemoglobin, and higher D-dimers in a small subset of patients supporting a pro-inflammatory and coagulopathic picture. Our study will add to a growing body of literature on SCD COVID-19 cases.
Darbari: Global Blood Therapeutics: Consultancy; Hilton Publishing Inc.: Consultancy; Novartis: Consultancy. Majumdar: Asklepion Pharma: Consultancy, Patents & Royalties: IV L-Citrulline in the use of sickle cell pain crisis. Campbell: Novartis Pharmaceuticals Corporation: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal