Abstract
Introduction
Invasive fungal infection (IFI) is a potentially devastating complication after allogeneic stem cell transplantation (aHSCT). Breakthrough infections are an increasing threat. The adoptive transfer of fungus-specific T cells (FSTs), analogous to virus specific T-cells for viral reactivation, may improve clinical outcomes but the delay involved in generating FSTs from the stem cell donor is problematic for this type of adoptive cell therapy. We created a bank of FSTs from normal donors for use in patients with post-transplant IFI. Here we describe the first use of partially HLA matched 3 rd party FSTs to treat IFI in a patient after aHSCT.
Methods
FSTs were manufactured by stimulating G-CSF primed apheresis products overnight with monocyte derived dendritic cells pulsed with lysates from Aspergillus terreus and Candida krusei. CD137 expressing cells were isolated and cultured with CD137 negative feeders in medium supplemented with IL-2, IL-7 and IL-15 for 12 days prior to assessment of target specificity and HLA restriction. Cells consisted principally of CD4 + lymphocytes secreting TNF-α, interferon-γ and IL-17 in response to fungal antigen stimulation. Standard release criteria were applied. We commenced a phase 1 cell dose escalation trial to assess the safety and efficacy of partially HLA-DR matched 3 rd party FSTs from a cryopreserved bank in patients with proven or probable IFI after aHSCT.
Results
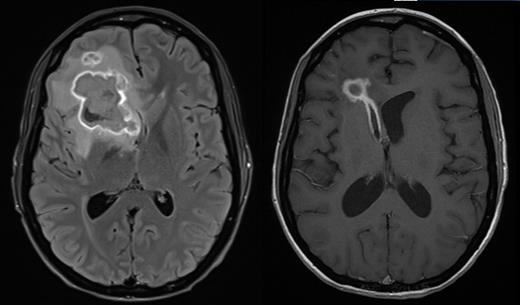
Patient A is a 27 year old female who underwent a myeloablative sibling haploidentical transplant for Philadelphia negative B-ALL in second remission. At day 100 she developed grade III lower GI GVHD requiring methylprednisolone and ruxolitinib for steroid dependent disease. Subsequent CMV reactivation was treated with ganciclovir. Cough in association with pulmonary infiltrates followed, and a bronchoalveolar lavage grew Scedosporium aurantiacum, Clavispora lusitaniaand Aspergillus fumigatus. She developed severe headache and MRI imaging showed a thin rim-enhancing 38x37 mm lesion within the right frontal lobe with moderate vasogenic oedema. Surgical drainage yielded 20 ml of frank pus that grew Scedosporium aurantiacum.
Antifungal therapy was started with vorinconazole, terbinafine, amphotericin and later caspofungin. Serial imaging after 12 days showed two new hyperdense foci representing extension of infection, consistent with worsening headaches. The patient received a single infusion of 3 rd party FSTs at a dose of 1 x10 6/m 2 at day 170 post-transplant while continuing antifungal therapy with vorinconazole and terbinafine. The product consisted of 94.1% CD4 +, 3.5% CD8 + T-cells and 1% NK cells and contained CD4 + T-cells responsive to both A. terreus and C. krusei antigens presented by HLA DR*03:01 shared between product and patient. There were no infusion related adverse events. Corticosteroids and calcineurin inhibitors were continued. Within one week of FST infusion there was an increase in the number of naïve and central memory CD4 + T-cells in blood and a fall in the number of CD4 + and CD8 + T-cells expressing Tim3. Over the following 3 months, there was a gradual rise in the number of CD4 + Tem and CD4 + Temra with a later and less pronounced rise in the analogous CD8 + populations. Serial imaging demonstrated rapid regression of the pulmonary abnormalities and gradual regression of the cerebral lesion at day 150 following FST infusion. 279 days after transplant and 109 days after infusion of FSTs, the patient developed worsening of headache and MRI confirmed rupture of the abscess into the right ventricle. Headache gradually resolved and the patient was discharged from hospital 329 days after transplant with ECOG 1 and no neurological abnormalities. However she was readmitted 13 days later with more severe headache with repeat imaging confirming raised intracranial pressure. CSF showed no evidence of fungi by PCR or culture. A CSF shunt was inserted and the patient remains well.
Conclusion
We report the first infusion of 3 rd party partially HLA DR matched fungus-specific T-cells for disseminated fungal infection following allogeneic stem cell transplantation. These data demonstrate the feasibility of this approach. The patient's favourable clinical outcome and phenotyping results suggest that the initial cell dose level is safe and may be associated with immune alterations that promote anti-fungal activity. Further trial recruitment is ongoing.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal