Abstract
INTRODUCTION: Many patients (pts) with newly diagnosed (ND) AML achieve remission with IC regimens, typically comprising cytarabine and an anthracycline, with the eventual goal of therapy being cure. On 21 Nov 2018, the FDA approved venetoclax (VEN), a BCL2 inhibitor, in combination with azacitidine (AZA), a hypomethylating agent, for treatment (Tx) of pts with ND AML aged ≥ 75 years or with comorbidities that preclude use of IC. The efficacy of the VEN-AZA regimen in ND AML was established based on a complete remission (CR) rate of 37%, and a composite CR + CR with incomplete hematologic recovery (CRh) rate of 66% (DiNardo, 2020). Subsequent randomized phase 3 trial data showed improvement in overall survival (OS) with VEN-AZA compared with AZA alone among older pts with AML unfit for IC, but a cure cannot currently be achieved with this regimen. It is important to understand the patterns of use of this combination relative to IC in adult pts with ND AML treated in real-world clinical practice, particularly when induction decisions for some pts can be challenging.
OBJECTIVE: To describe real-world CR rates, OS, and relapse-free survival (RFS) achieved with IC (induction ± consolidation) and VEN-AZA as first-line therapy for ND AML.
METHODS: This retrospective cohort from the US-based Flatiron Health electronic medical records (EMR) database included pts with ND AML who received induction with VEN-AZA or IC between 21 Nov 2018 and 30 Nov 2020 and had ≥ 2 months of follow-up. Pts were separated into 3 age-based subgroups: 18-59, 60-75, and > 75 years. Assessments included the CR rate; RFS, defined as the time from start of therapy to relapse or death; and OS, the time from start of therapy until death. Both OS and RFS were estimated by Kaplan-Meier Methods.
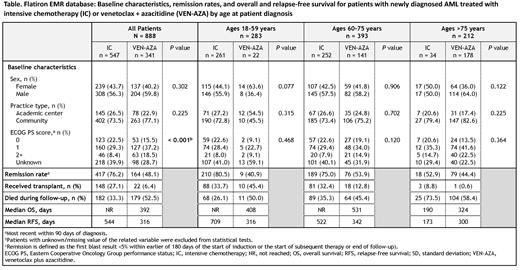
RESULTS: In all, 888 pts met the selection criteria, including 547 pts (61.6%) who received IC and 341 (38.4%) who received VEN-AZA. Baseline characteristics were generally comparable between cohorts with respect to sex (57.7% male overall) and practice type (74.9% treated at a community practice), but as expected, pts in the VEN-AZA cohort were significantly older than those in the IC cohort (mean [SD] age 58.1 [13.9] vs. 73.8 [9.1] years, respectively; P < 0.001). The age-based subgroups comprised 283 pts (31.9%) aged 18-59 years (IC, n = 261;VEN-AZA, n = 22), 393 pts (44.3%) aged 60-75 y (IC, n = 252; VEN-AZA, n = 141), and 212 pts (23.8%) aged > 75 years (IC, n = 34; VEN-AZA, n = 178) (Table). Thus, the 60-75 years pt subgroup comprised the most pts; few pts in the 18-59 years subgroup received VEN-AZA and few pts in the > 75 years subgroup received IC.
Overall, the CR rate was 76.2% (417/547) in the IC cohort and 48.1% (164/341) in the VEN-AZA cohort (Table). Remission rate in the age 60-75 years subgroup was 75.0% (189/252) in pts who received IC and 53.9% (76/141) in pts who received VEN-AZA. The proportions of pts aged 60-75 who underwent transplant were 32.4% in the IC cohort and 12.8% in the VEN-AZA cohort.
Median follow-up from diagnosis was 365 days in the IC cohort and 292 days in the VEN-AZA cohort. During the follow-up periods, the overall mortality rates for patients aged 60-75 years treated with IC and VEN-AZA were 35.3% (89/252) and 45.4% (64/141), respectively. Among all pts, estimated OS in IC-treated pts was not reached (NR) and in the VEN-AZA cohort was 392 days, and was NR and 531 days, respectively, in the subgroup of pts aged 60-75 years (Table). Among all pts, estimated median RFS was 544 days in the IC cohort and was 316 days in the VEN-AZA cohort; within the subgroup of pts aged 60-75 years, median RFS was 522 days and 342 days, respectively.
CONCLUSIONS: As expected, use of IC was more common than VEN-AZA in pts aged ≤ 75 years, but approximately one-third of pts aged 60-75 years received VEN-AZA. IC was associated with nominally better remission rate and survival compared with VEN-AZA among pts aged ≤ 75 years. An ongoing analysis will use propensity score matching (or other appropriate method) to adjust for baseline covariates to identify predictors of VEN-AZA use, and to compare the impact of IC vs. VEN-AZA on OS. Data from that analysis will be presented at the congress.
Zeidan: Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Geron: Other: Clinical Trial Committees; Jazz: Consultancy; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Epizyme: Consultancy; Ionis: Consultancy; Boehringer Ingelheim: Consultancy, Research Funding; Astellas: Consultancy; Janssen: Consultancy; Gilead: Consultancy, Other: Clinical Trial Committees; Aprea: Consultancy, Research Funding; Genentech: Consultancy; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding; Pfizer: Other: Travel support, Research Funding; Acceleron: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; ADC Therapeutics: Research Funding; AstraZeneca: Consultancy; Agios: Consultancy; BioCryst: Other: Clinical Trial Committees; BeyondSpring: Consultancy; Astex: Research Funding; Daiichi Sankyo: Consultancy; Incyte: Consultancy, Research Funding; Jasper: Consultancy; Kura: Consultancy, Other: Clinical Trial Committees; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding. Pollyea: Syndax: Honoraria, Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other; Syros: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: advisory board; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aprea: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Other: advisory board; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kiadis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Other: advisory board; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva: Research Funding; Curis, Servier: Other; Pfizer: Research Funding; Agios: Other, Research Funding. Borate: Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharma: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy. Vasconcelos: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Potluri: Bristol Myers Squibb: Consultancy. Rotter: SmartAnalyst Inc.: Current Employment. Gaugler: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bonifacio: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Chen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal